��Ŀ����
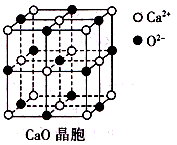
X��Y��Z��ԭ���������ε����Ķ�����Ԫ�أ�3��Ԫ�ص�ԭ�Ӻ��������֮����Ca2���ĺ����������ȣ�X��Z�ֱ�õ�һ�����Ӻ���γ�ϡ������ԭ�ӵ��ȶ����Ӳ�ṹ������˵����ȷ����
| A��ԭ�Ӱ뾶��Z��Y��X |
| B��Z��X�γɻ�����ķе����Z��ͬ��Ԫ����X�γɻ�����ķе� |
| C��CaY2��ˮ����������ԭ��Ӧʱ��CaY2ֻ�������� |
| D��CaX2��CaY2��CaZ2��3�ֻ������У��������������Ӹ����Ⱦ�Ϊ1�U2 |
B
Ca2+�ĺ��������Ϊ18��X���ڷֱ�õ�һ�����Ӻ���γ�ϡ������ԭ�ӵ��ȶ����Ӳ�ṹ˵��X��Z���γɸ�1�����ӣ���XΪH��ZΪF����X������ΪF��Z������ΪCl���ɴ˿ɽ�һ���Ƴ�YΪO��ԭ�Ӱ뾶��O>F>H��A�����HF���Ӽ�������������е���ͬ����Ԫ���⻯������ߵģ�B����ȷ��CaO2��ˮ������Ӧʱ��O22���绯��������������������ԭ����C�����CaO2�е��������������Ӹ����Ⱦ�Ϊ1:1��D�����
�����㶨λ�����⿼�������Ԫ�ص������ƶϺ�Ԫ�����ڱ�֪ʶ��
�����㶨λ�����⿼�������Ԫ�ص������ƶϺ�Ԫ�����ڱ�֪ʶ��

��ϰ��ϵ�д�
 ��У����ϵ�д�
��У����ϵ�д�
�����Ŀ
 ��ÿ�������з�̯2����ԭ��
��ÿ�������з�̯2����ԭ�� Rx[CrCln(H2O)6��n]+xH+������0.0015 mol[CrCln(H2O)6��n]x������Һ����R-H��ȫ�������к����ɵ�H+��Ũ��Ϊ0.1200 mol��L-1NaOH��Һ25.00 mL����������ӵĻ�ѧʽΪ_______��
Rx[CrCln(H2O)6��n]+xH+������0.0015 mol[CrCln(H2O)6��n]x������Һ����R-H��ȫ�������к����ɵ�H+��Ũ��Ϊ0.1200 mol��L-1NaOH��Һ25.00 mL����������ӵĻ�ѧʽΪ_______��