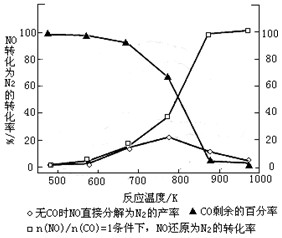
��Ŀ����
�ڸ��¸�ѹ��CO���м��ߵĻ�ѧ���ԣ�������ֵ��ʻ��ﷴӦ��
��1�����ں��º��ݵ������ڽ��з�ӦC(s)+H2O(g) CO(g)+H2(g)����������жϸ÷�Ӧ�ﵽƽ��״̬�ı�־�� ��(����ĸ)
CO(g)+H2(g)����������жϸ÷�Ӧ�ﵽƽ��״̬�ı�־�� ��(����ĸ)
a�������ڵ�ѹǿ���ֲ��� b��������H2Ũ����COŨ�����
c�������л��������ܶȱ��ֲ��� d��CO������������H2�������������
��2��COһ����ȼ�ϵ����ʹ�õĵ�����Dz�����Y2O3��ZrO2���壬���ڸ������ܴ���O2���õ�ظ����ĵ缫��ӦʽΪ ��
��3��һ�������£�CO��H2�ɺϳɼ��飬��Ӧ����ʽΪ��CO(g)+3H2(g) CH4(g)+ H2O (g)
CH4(g)+ H2O (g)
��һ�������£��÷�Ӧ�ܹ��Է����е�ԭ���� ��
����֪H2(g)��CO(g)�� CH4(g)��ȼ���ȷֱ�Ϊ285��8 kJ��mol-1��283��0 kJ��mol-1��890,0 kJ��mol-1��
д��CO��H2��Ӧ����CH4��CO2���Ȼ�ѧ����ʽ�� ��
��4����ҵ�Ͽ�ͨ���״��ʻ�������ȡ�����������Ӧ����ʽΪ�� ��
CH3OH(g)+CO(g) HCOOCH3(g) ��H=��29.1 kJ��mol-1
HCOOCH3(g) ��H=��29.1 kJ��mol-1
������Ա�Ը÷�Ӧ�������о��������о�������£�
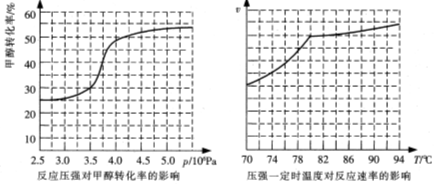
�ٴӷ�Ӧѹǿ�Լ״�ת���ʵ�Ӱ�조Ч��"������ҵ��ȡ�������Ӧѡ���ѹǿ�� ��
��ʵ�ʹ�ҵ�����в��õ��¶���80�棬�������� ��
��1�����ں��º��ݵ������ڽ��з�ӦC(s)+H2O(g)
 CO(g)+H2(g)����������жϸ÷�Ӧ�ﵽƽ��״̬�ı�־�� ��(����ĸ)
CO(g)+H2(g)����������жϸ÷�Ӧ�ﵽƽ��״̬�ı�־�� ��(����ĸ)a�������ڵ�ѹǿ���ֲ��� b��������H2Ũ����COŨ�����
c�������л��������ܶȱ��ֲ��� d��CO������������H2�������������
��2��COһ����ȼ�ϵ����ʹ�õĵ�����Dz�����Y2O3��ZrO2���壬���ڸ������ܴ���O2���õ�ظ����ĵ缫��ӦʽΪ ��
��3��һ�������£�CO��H2�ɺϳɼ��飬��Ӧ����ʽΪ��CO(g)+3H2(g)
 CH4(g)+ H2O (g)
CH4(g)+ H2O (g)��һ�������£��÷�Ӧ�ܹ��Է����е�ԭ���� ��
����֪H2(g)��CO(g)�� CH4(g)��ȼ���ȷֱ�Ϊ285��8 kJ��mol-1��283��0 kJ��mol-1��890,0 kJ��mol-1��
д��CO��H2��Ӧ����CH4��CO2���Ȼ�ѧ����ʽ�� ��
��4����ҵ�Ͽ�ͨ���״��ʻ�������ȡ�����������Ӧ����ʽΪ�� ��
CH3OH(g)+CO(g)
 HCOOCH3(g) ��H=��29.1 kJ��mol-1
HCOOCH3(g) ��H=��29.1 kJ��mol-1������Ա�Ը÷�Ӧ�������о��������о�������£�

�ٴӷ�Ӧѹǿ�Լ״�ת���ʵ�Ӱ�조Ч��"������ҵ��ȡ�������Ӧѡ���ѹǿ�� ��
��ʵ�ʹ�ҵ�����в��õ��¶���80�棬�������� ��
��1��ac
��2��COһ2eһ+O2һ=CO2
��3���ٸ÷�Ӧ��H<0
��2CO(g)+2H2(g)
 CH4(g)+C02(g) ��H="-247.6" kJ��mol-1
CH4(g)+C02(g) ��H="-247.6" kJ��mol-1��4����3.5��106 Pa��4.0��106 Pa
�ڸ���80��ʱ���¶ȶԷ�Ӧ����Ӱ���С���ҷ�Ӧ���ȣ������¶�ʱƽ�������ƶ���ת���ʽ���
(ÿ��2�֣����12��)
�����������1�������������ƽ�⡱���淴Ӧ������ϵ��ѹǿ���ܶȣ���������������仯�����DZ�����b�����ʵ�Ũ�Ȳ�����ƽ���־�������ֻ��һ���ض�״̬�������ԣ�d��CO������������H2�������������ͬ��һ����ȣ���2������COʧȥ���ӣ�����CO2��COһ2eһ+O2һ=CO2 ����3���ٸ÷�Ӧ�ġ�S<0��ֻ�ܡ�H<0�����Է����У���2CO(g)+2H2(g)
 CH4(g)+C02(g) ��H="-247.6" kJ��mol-1����4������ͼ��֪����ѹǿ�ﵽ4.0��106 Paѹǿ��ת���ʵ�Ӱ���С���ʹ�ҵ��ȡ�������Ӧѡ���ѹǿ��3.5��106 Pa��4.0��106 Pa������ͼ�۲��֪�¶ȸ���80��ʱ���¶ȶԷ�Ӧ����Ӱ���С���ҷ�Ӧ���ȣ������¶�ʱƽ�������ƶ���ת���ʽ��͡�
CH4(g)+C02(g) ��H="-247.6" kJ��mol-1����4������ͼ��֪����ѹǿ�ﵽ4.0��106 Paѹǿ��ת���ʵ�Ӱ���С���ʹ�ҵ��ȡ�������Ӧѡ���ѹǿ��3.5��106 Pa��4.0��106 Pa������ͼ�۲��֪�¶ȸ���80��ʱ���¶ȶԷ�Ӧ����Ӱ���С���ҷ�Ӧ���ȣ������¶�ʱƽ�������ƶ���ת���ʽ��͡�
��ϰ��ϵ�д�
�����Ŀ

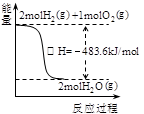
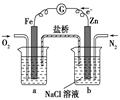
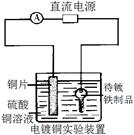
 ��������������ʱ�����������Ӧ����v��SO2����SO2ת���ʾ�����
��������������ʱ�����������Ӧ����v��SO2����SO2ת���ʾ�����



 CO2(g)��H2(g)���õ������������ݣ�
CO2(g)��H2(g)���õ������������ݣ�
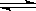 CH3OH(g) ��H1����116 kJ��mol-1
CH3OH(g) ��H1����116 kJ��mol-1

 CH3CH2OH(g)+H2O(g) ��H=��256.1kJ��mol��1��
CH3CH2OH(g)+H2O(g) ��H=��256.1kJ��mol��1�� CO2(g)+H2(g) ��H=��41.2kJ��mol��1
CO2(g)+H2(g) ��H=��41.2kJ��mol��1