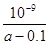��Ŀ����
�о�̼���仯��������ʶԴٽ���̼���Ĺ���������Ҫ���塣
(1)����ͬ����CO(g)��H2O(g)�ֱ�ͨ�����Ϊ2L�ĺ����ܱ������У����з�Ӧ CO(g)��H2O(g) CO2(g)��H2(g)���õ������������ݣ�
CO2(g)��H2(g)���õ������������ݣ�
��ʵ��1����CO2��ʾ�ķ�Ӧ����Ϊ_____________(����2λС��)��
�ڸ÷�ӦΪ___________(����ȡ����ȡ�)��Ӧ��
��ʵ��2��ƽ�ⳣ��Ϊ___________________________��
(2)��֪�ڳ��³�ѹ�£�
2CH3OH(l)+3O2(g)��2CO2(g)+4H2O(l) ��H����1451.6kJ/mol
2CO(g)+O2(g)��2CO2(g) ��H����566.0kJ/mol
д���״�����ȫȼ������CO��Һ̬ˮ���Ȼ�ѧ����ʽ_______________________________��
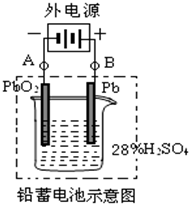
(3)�״���ˮ�ʻ����һ������Ⱦ����һ�ֵ绯ѧ��������������Ⱦ��ʵ��������ͼװ��ģ��ù��̣���ԭ���ǣ�ͨ���Co2+��������Co3+��Ȼ����Co3+���������Ѽ״�������CO2����ȥ(Co3+�Ļ�ԭ������CO2+)��
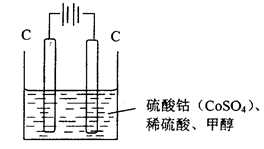
��д�������缫��Ӧʽ_______________________________________________________________ ��
��д����ȥ�״������ӷ���ʽ__________________________________________________________��
(1)����ͬ����CO(g)��H2O(g)�ֱ�ͨ�����Ϊ2L�ĺ����ܱ������У����з�Ӧ CO(g)��H2O(g)
 CO2(g)��H2(g)���õ������������ݣ�
CO2(g)��H2(g)���õ������������ݣ�| ʵ���� | �¶ȡ� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min | ||
| CO | H2O | H2 | CO | |||
| 1 | 650 | 4 | 2 | 1.6 | 2.4 | 6 |
| 2 | 900 | 2 | 1 | 0.4 | 1.6 | 3 |
�ڸ÷�ӦΪ___________(����ȡ����ȡ�)��Ӧ��
��ʵ��2��ƽ�ⳣ��Ϊ___________________________��
(2)��֪�ڳ��³�ѹ�£�
2CH3OH(l)+3O2(g)��2CO2(g)+4H2O(l) ��H����1451.6kJ/mol
2CO(g)+O2(g)��2CO2(g) ��H����566.0kJ/mol
д���״�����ȫȼ������CO��Һ̬ˮ���Ȼ�ѧ����ʽ_______________________________��
(3)�״���ˮ�ʻ����һ������Ⱦ����һ�ֵ绯ѧ��������������Ⱦ��ʵ��������ͼװ��ģ��ù��̣���ԭ���ǣ�ͨ���Co2+��������Co3+��Ȼ����Co3+���������Ѽ״�������CO2����ȥ(Co3+�Ļ�ԭ������CO2+)��

��д�������缫��Ӧʽ_______________________________________________________________ ��
��д����ȥ�״������ӷ���ʽ__________________________________________________________��
��12�֣���1����0.13mol/��L?min����2�֣� �ڷ��ȣ�1�֣� ��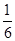 ��2�֣�
��2�֣�
��2��CH3OH(l)��O2(g)��CO(g)��2H2O(l) ��H����442.8kJ/mol ��3�֣�����ʽ2�֣���ֵ1�֣�
��3����Co2+��e����Co3+��2�֣� ��6Co3+��CH3OH��H2O��CO2����6Co2+��6H+��2�֣�
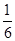 ��2�֣�
��2�֣���2��CH3OH(l)��O2(g)��CO(g)��2H2O(l) ��H����442.8kJ/mol ��3�֣�����ʽ2�֣���ֵ1�֣�
��3����Co2+��e����Co3+��2�֣� ��6Co3+��CH3OH��H2O��CO2����6Co2+��6H+��2�֣�
�����������1���ٸ��ݱ������ݿ�֪�����������ʵ����仯��Ϊ1.6mol����˸��ݷ�Ӧ�ķ���ʽ��֪��CO2�ı仯��Ҳ��1.6mol����Ũ����1.6mol��2L��0.8mol/L������CO2��ʾ�ķ�Ӧ������v(CO2)��0.8mol/L��6min��0.13mol/��L?min����
�ڸ��ݱ������ݿ�֪��ʵ��1��CO��ת����Ϊ
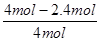 ��100%��40%��ʵ��2��CO��ת����Ϊ
��100%��40%��ʵ��2��CO��ת����Ϊ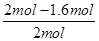 ��100%��20%�����ʵ��1��ת���ʴ���ʵ��2����˵���¶�����ƽ�����淴Ӧ�����ƶ����������Ӧ���ȡ�
��100%��20%�����ʵ��1��ת���ʴ���ʵ��2����˵���¶�����ƽ�����淴Ӧ�����ƶ����������Ӧ���ȡ���ƽ��ʱCO�����ʵ���Ϊ1.6mol��2L��0.8mol/L����
CO(g)��H2O(g)
 CO2(g)��H2(g)
CO2(g)��H2(g)��ʼŨ�ȣ�mol/L�� 1 0.5 0 0
ת��Ũ�ȣ�mol/L�� 0.2 0.2 0.2 0.2
ƽ��Ũ�ȣ�mol/L�� 0.8 0.3 0.2 0.2
��ѧƽ�ⳣ������һ�������£������淴Ӧ�ﵽƽ��״̬ʱ��������Ũ�ȵ���֮���ͷ�Ӧ��Ũ�ȵ���֮���ı�ֵ.����900��ʱ�÷�Ӧƽ�ⳣ��K��
 ��
��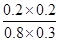 ��0.17��
��0.17����2����֪��Ӧ��2CH3OH(l)+3O2(g)��2CO2(g)+4H2O(l) ��H����1451.6kJ/mol�ͷ�Ӧ��2CO(g)+O2(g)��2CO2(g) ��H����566.0kJ/mol������ݸ�˹���ɿ�֪������-�ڣ���2����CH3OH(l)��O2(g)��CO(g)��2H2O(l)�����Ը÷�Ӧ�ķ�Ӧ�ȡ�H������1451.6kJ/mol��566.0kJ/mol����2����442.8kJ/mol��
��3���ٵ���������ʧȥ���ӣ�����������Ӧ�����ͨ���Co2+������ʧȥ����������Co3+�����������缫��ӦʽΪCo2+��e����Co3+��
����Co3+����������ˮ�еļ״�������CO2����ȥ����������ԭΪCo2+�����ԭ���غ������غ��֪���÷�Ӧ�����ӷ���ʽΪ6Co3+��CH3OH��H2O��CO2����6Co2+��6H+��

��ϰ��ϵ�д�
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
�����Ŀ
 H2(g)+I2(g)ƽ����ϵ������ƽ����ϵ��ѹǿ��ʹ��ɫ���������������ԭ�����͡�
H2(g)+I2(g)ƽ����ϵ������ƽ����ϵ��ѹǿ��ʹ��ɫ���������������ԭ�����͡� CO(g)+H2(g)����������жϸ÷�Ӧ�ﵽƽ��״̬�ı�־�� ��(����ĸ)
CO(g)+H2(g)����������жϸ÷�Ӧ�ﵽƽ��״̬�ı�־�� ��(����ĸ) HCOOCH3(g) ��H=��29.1 kJ��mol-1
HCOOCH3(g) ��H=��29.1 kJ��mol-1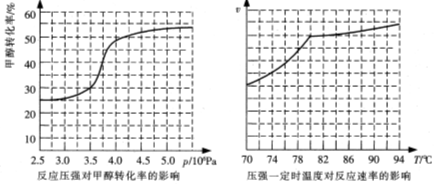
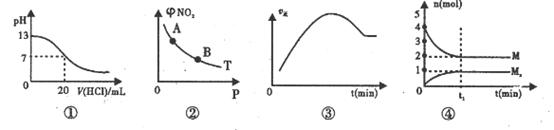
 N2O4(g)�ﵽƽ��ʱNO2���������
N2O4(g)�ﵽƽ��ʱNO2��������� (NO2)��ѹǿP�ı仯��ͼ����ʾ����A�����ɫ�B�����ɫdz
(NO2)��ѹǿP�ı仯��ͼ����ʾ����A�����ɫ�B�����ɫdz ������
������
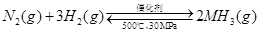 ��H=��38.6kJ��mol-1
��H=��38.6kJ��mol-1