��Ŀ����
����Ŀ����ѧ�Ҷ�һ̼��ѧ�����˹㷺������о���ȡ����һЩ��Ҫ�ɹ���
��1����֪��CO(g)+2H2(g) ![]() CH3OH(g) ��H1����90.0kJ/mol��
CH3OH(g) ��H1����90.0kJ/mol��
3CH3OH(g) ![]() CH3CH=CH2(g)+3H2O(g) ��H2����31.0kJ/mol
CH3CH=CH2(g)+3H2O(g) ��H2����31.0kJ/mol
CO��H2�ϳ�CH3CH=CH2���Ȼ�ѧ����ʽΪ________��
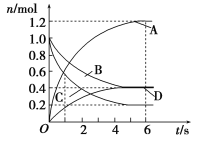
��2���״���CH3OH������Ϊ������������ȼ�ϣ���ҵ�Ͽ���CO�� H2�ڴ��������ºϳɼ״����������Ϊ2L�ĺ��ݾ����ܱ������У�����1molCO ��2mo1H2������Ӧ��CO(g)+2H2(g) ![]() CH3OH(g) ��H1��-90.0kJ/mol������Ӧ���е�5minʱ�ﵽƽ��״̬����ʱCH3OH�����ʵ���Ϊ0.6mol����
CH3OH(g) ��H1��-90.0kJ/mol������Ӧ���е�5minʱ�ﵽƽ��״̬����ʱCH3OH�����ʵ���Ϊ0.6mol����
��5 min�ڷ�Ӧ��ƽ��������(H2) = _____ mol/(L��min)��
�ڴﵽƽ��ʱ�ų�������Ϊ________ kJ
�۲���˵���÷�Ӧ�Ѵﵽƽ��״̬����____��ѡ����ĸ��ţ�
a��CO�����ʵ������ٸı� b���������¶ȱ��ֲ���
c��CH3OH����������������������� d�������ڵ��ܶȱ��ֲ���
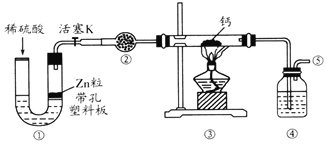
��3��һ�ּ״�ȼ�ϵ����ͼ��ʹ�õĵ������Һ��2mol��L��1��KOH��Һ��
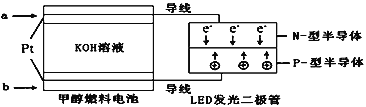
��д������(ͨ��)a����һ���ĵ缫��Ӧʽ_____��ÿ����9.6g�״�ת�Ƶĵ�����Ϊ_______��
���𰸡�3CO(g)+6H2(g) CH3CH=CH2(g)+3H2O(g)��H��-301.0kJ/mol 0.12 54 d CH3OH��6e��+8OH��=CO32-+6H2O 1.8NA(1.8��6.02��1023)
��������
(1)��CO(g)+2H2(g)![]() CH3OH(g)��H1=-90.1kJ/mol����3CH3OH(g)
CH3OH(g)��H1=-90.1kJ/mol����3CH3OH(g)![]() CH3CH=CH2(g)+3H2O(g)��H2=-31.0kJ/mol����˹���ɼ������3+�ڵõ�CO��H2�ϳ�CH3CH=CH2���Ȼ�ѧ����ʽ��
CH3CH=CH2(g)+3H2O(g)��H2=-31.0kJ/mol����˹���ɼ������3+�ڵõ�CO��H2�ϳ�CH3CH=CH2���Ȼ�ѧ����ʽ��
(2)����Ӧ���е�5minʱ�ﵽƽ��״̬����ʱCH3OH�����ʵ���Ϊ0.6mol����Ϸ�ӦCO(g)+2H2(g) ![]() CH3OH(g)��֪�μӷ�Ӧ��H2�����ʵ���Ϊ1.2mol��
CH3OH(g)��֪�μӷ�Ӧ��H2�����ʵ���Ϊ1.2mol��
��5 min�ڷ�Ӧ��ƽ��������(H2) =![]() ��
��
�ڽ���Ȼ�ѧ��Ӧ����ʽ����ﵽƽ��ʱ�ų���������
�۸��ݻ�ѧƽ��״̬���������淴Ӧ������ȣ�����ֺ������ֲ��������
(3)���ݵ��ӷ���aΪ������Ϊ�״�����������Ӧ���ɶ�����̼���ݴ���д���ɵ缫��Ӧ��֪��ÿ����1mol�״�ת�Ƶ���6mol��9.6g�״�Ϊ![]() =0.3mol���ݴ˼��㡣
=0.3mol���ݴ˼��㡣
(1)��CO(g)+2H2(g)CH3OH(g)��H1=-90.1kJ/mol����3CH3OH(g)CH3CH=CH2(g)+3H2O(g)��H2=-31.0kJ/mol����˹���ɼ������3+�ڵõ�CO��H2�ϳ�CH3CH=CH2���Ȼ�ѧ����ʽ��3CO(g)+6H2(g)CH3CH=CH2(g)+3H2O(g)��H=-301.3kJ/mol��
(2)����Ӧ���е�5minʱ�ﵽƽ��״̬����ʱCH3OH�����ʵ���Ϊ0.6mol����Ϸ�ӦCO(g)+2H2(g) ![]() CH3OH(g)��֪�μӷ�Ӧ��H2�����ʵ���Ϊ1.2mol��
CH3OH(g)��֪�μӷ�Ӧ��H2�����ʵ���Ϊ1.2mol��
��5 min�ڷ�Ӧ��ƽ��������(H2) =![]() =
= =0.12 mol/(L��min)��
=0.12 mol/(L��min)��
�ڲμӷ�Ӧ��H2�����ʵ���Ϊ1.2mol����ƽ��ʱ�ų�������Ϊ1.2mol![]() =54kJ��
=54kJ��
��a����Ӧ�ﵽƽ�⣬CO�����ʵ������ٸı䣬��a��ȷ��
b���������¶ȱ��ֲ��䣬˵����Ӧ������Ծ�ֹ״̬����ƽ��״̬����b��ȷ��
c��CH3OH����������������������ȣ�˵��CH3OH�������ٸı䣬�ﵽƽ�⣬��c��ȷ��
d��������=![]() ����Ӧ��Ϊ���壬�������������䣬�������ݣ�V���䣬�������ڵ��ܶ�ʼ�ձ��ֲ��䣬�����ж�ƽ�⣬��d����
����Ӧ��Ϊ���壬�������������䣬�������ݣ�V���䣬�������ڵ��ܶ�ʼ�ձ��ֲ��䣬�����ж�ƽ�⣬��d����
�ʴ�Ϊ��d��
(3)������a������aΪ����������Ϊ�״�ʧȥ���ӷ���������Ӧ���缫��ӦΪ��CH3OH-6e-+8OH-=CO32-+6H2O���ɵ缫��Ӧ��֪��ÿ����1mol�״�ת�Ƶ���6mol��ÿ����9.6g�״�ת�Ƶĵ�����Ϊ![]() ��6��NA=1.8NA(1.8��6.02��1023)��
��6��NA=1.8NA(1.8��6.02��1023)��

����Ŀ����ͼ��Ԫ�����ڱ���һ���֣�
���ݱ�� | ����NaOH��Һ����� | ��Һ��pH | |
HX | HZ | ||
1 | 0 | 3 | 1 |
2 |
| a | b |
![]() ʱ����Ũ��Ϊ
ʱ����Ũ��Ϊ![]() ������������Һ�ֱ�ζ�
������������Һ�ֱ�ζ�![]() Ũ�Ⱦ�Ϊ
Ũ�Ⱦ�Ϊ![]() ��������HX��
��������HX��![]() ��������仯
��������仯![]() ��ʵ���������2�������ж���ȷ����
��ʵ���������2�������ж���ȷ����![]()
A.ͨ�������ɵñ�����![]() ��
��![]()
B.��������HZ��Һϡ��100����pH��HX��Һ��pH��
C.����ZԪ�صķǽ����Ա�Yǿ������Z�⻯������Խ�ǿ
D.![]()
![]()
![]() ��ˮ��Һ�У�
��ˮ��Һ�У�![]()
![]()