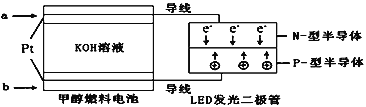
��Ŀ����
����Ŀ����t��ʱ��ijNaOHϡ��Һ�У�c(H+)=10-amol/L��c(OH-)=10-bmol/L����֪a+b=12����
��1�����¶��£�ˮ�����ӻ�����Kw=___��
��2�����¶��£���100mL0.1mol/L��ϡ������100mL0.4mol/L��NaOH��Һ��Ϻ���Һ��pH=___����ʱ����Һ����ˮ�����c(OH-)=___mol/L��
��3�������£�pH=3��������Һ��pH=11��NaOH��Һ�������Ϻ����Һ��pH___����>7��=7��<7����pH=3�Ĵ�����Һ��pH=11��NaOH��Һ�������Ϻ����Һ��pH___����>7��=7��<7����
���𰸡�1��10-12 11 1��10-11mol/L =7 <7
��������
��t��ʱ��ijNaOHϡ��Һ�У�c(H+)=10-amol/L��c(OH-)=10-bmol/L����֪a+b=12����
��1�����¶��£�ˮ�����ӻ�����Kw= c(H+) c(OH-)���������ݣ��������Kw��
��2�����¶��£���100mL0.1mol/L��ϡ������100mL0.4mol/L��NaOH��Һ��Ϻ����Һ�У�c(OH-)=![]() ������Kw�������Һ�е�c(H+)���Ӷ������Һ��pH����ʱ����Һ����ˮ�����c(OH-)= c(H+)��
������Kw�������Һ�е�c(H+)���Ӷ������Һ��pH����ʱ����Һ����ˮ�����c(OH-)= c(H+)��
��3�������£�pH=3��������Һ��pH=11��NaOH��Һ�����(����Ϊ1L)��Ϻ�������������n(H+)=10-3mol/L��1L=10-3mol��NaOH���������n(OH-)=10-3mol/L��1L=10-3mol�����߸պ���ȫ��Ӧ�������Һ�����ԣ�pH=3�Ĵ�����Һ��pH=11��NaOH��Һ�������Ϻ���ȻH+��OH-�����ʵ���Ҳ��ȣ������ڴ�����Һ�д��ڴ���δ����Ĵ��ᣬ���Է�Ӧ�������ʣ�࣬��������ʹ��Һ�����ԡ�
��t��ʱ��ijNaOHϡ��Һ�У�c(H+)=10-amol/L��c(OH-)=10-bmol/L����֪a+b=12����
��1�����¶��£�ˮ�����ӻ�����Kw= c(H+) c(OH-)=10-a10-b=10-(a+b)=10-12����Ϊ��1��10-12��
��2�����¶��£���100mL0.1mol/L��ϡ������100mL0.4mol/L��NaOH��Һ��Ϻ����Һ�У�c(OH-)=![]() ������Kw�������Һ�е�c(H+)=
������Kw�������Һ�е�c(H+)=![]() =10-11mol/L���Ӷ������Һ��pH=11����ʱ����Һ����ˮ�����c(OH-)= c(H+)=10-11mol/L����Ϊ��11��1��10-11mol/L��
=10-11mol/L���Ӷ������Һ��pH=11����ʱ����Һ����ˮ�����c(OH-)= c(H+)=10-11mol/L����Ϊ��11��1��10-11mol/L��
��3�������£�pH=3��������Һ��pH=11��NaOH��Һ�����(����Ϊ1L)��Ϻ�������������n(H+)=10-3mol/L��1L=10-3mol��NaOH���������n(OH-)=10-3mol/L��1L=10-3mol�����߸պ���ȫ��Ӧ�������Һ�����ԣ�pH=7��pH=3�Ĵ�����Һ��pH=11��NaOH��Һ�������Ϻ���ȻH+��OH-�����ʵ���Ҳ��ȣ������ڴ�����Һ�д��ڴ���δ����Ĵ��ᣬ���Է�Ӧ�������ʣ�࣬��������ʹ��Һ�����ԣ�pH<7����Ϊ��=7��<7��

����Ŀ����֪��H2(g)+I2(g)![]() 2HI(g)����H= -14.9kJ��mol-1��ij�¶����ڼס������������ܱ������г��뷴Ӧ�����ʼŨ�����±���ʾ�����з�Ӧ�ﵽƽ��ʱ�����c(H2)=0.008mol��L-1�������ж���ȷ��
2HI(g)����H= -14.9kJ��mol-1��ij�¶����ڼס������������ܱ������г��뷴Ӧ�����ʼŨ�����±���ʾ�����з�Ӧ�ﵽƽ��ʱ�����c(H2)=0.008mol��L-1�������ж���ȷ��
��ʼŨ�� | c(H2)/(mol��L-1) | c(I2)/(mol��L-1) | c(HI)(mol��L-1) |
�� | 0.01 | 0.01 | 0 |
�� | 0.02 | 0.02 | 0 |
A.ƽ��ʱ������H2��ת�����Ǽ��е�2��
B.ƽ��ʱ�����л�������ɫ��������
C.ƽ��ʱ���ס����������ı仯ֵ���
D.���¶��£���Ӧ��ƽ�ⳣ��K=0.25
����Ŀ��ijʵ��С����H2O2�ֽ�Ϊ�����о�Ũ�ȡ���������Һ����ԶԷ�Ӧ���ʵ�Ӱ�졣�ڳ����°������·������ʵ�顣
ʵ���� | ��Ӧ�� | ���� |
�� | 10mL2%H2O2��Һ | �� |
�� | 10mL5%H2O2��Һ | �� |
�� | 10mL5%H2O2��Һ | 1mL0.1mol��L��1FeCl3��Һ |
�� | 10mL5%H2O2��Һ������HCl��Һ | 1mL0.1mol��L��1FeCl3��Һ |
�� | 10mL5%H2O2��Һ������NaOH��Һ | 1mL0.1mol��L��1FeCl3��Һ |
��1��ʵ��ٺ͢ڵ�Ŀ����___��
��2��ʵ��ۢܢ��У�������������������ʱ��仯�Ĺ�ϵ��ͼ1������ͼ1�ܹ��ó���ʵ�������__��

��3������0.1gMnO2��ĩ��50mLH2O2��Һ�У��ڱ�״���·ų�����������ʱ��Ĺ�ϵ��ͼ2��ʾ����Ӧ���ʱ仯��ԭ����__��