��Ŀ����
4�� ��֪25��ʱ����������ʵĵ���ƽ�ⳣ�����������ʾ��
��֪25��ʱ����������ʵĵ���ƽ�ⳣ�����������ʾ��| ��ѧʽ | CH3COOH | H2CO3 | HClO | |
| ����ƽ�ⳣ�� | Ka=1.8��10-5 | Ka1=4.3��10-7 | Ka2=5.6��10-11 | Ka=3.0��10-8 |
��1�����ʵ���Ũ�Ⱦ�Ϊ0.1mol•L-1��������Һ��
a��CH3COONa b��Na2CO3 c��NaClO d��NaHCO3
pH��С��������˳����a��d��c��b���ñ����д����
��2�������£�0.1mol/L��CH3COOH��Һ��ˮϡ�����У����б���ʽ�����ݱ�����BD��
A��c��H+�� B��$\frac{c��{H}^{+}��}{c��C{H}_{3}COOH��}$ C��c��H+��•c��OH-�� D��$\frac{c��O{H}^{-}��}{c��{H}^{+}��}$ E��$\frac{c��{H}^{+}��•c��C{H}_{3}CO{O}^{-}��}{c��C{H}_{3}COOH��}$
��3�������Ϊ100mL pH=2��CH3COOH��һԪ��HX����ˮϡ������pH����Һ����Ĺ�ϵ��ͼ��ʾ����HX�ĵ���ƽ�ⳣ�����ڣ�����ڡ�����С�ڡ����ڡ���CH3COOH�ĵ���ƽ�ⳣ���������Ǽ�ˮϡ����ͬ������һԪ���pH�仯Խ������Խǿ������ƽ�ⳣ��Խ��
���� ��1������ĵ���ƽ�ⳣ��Խ��������Խǿ���������ˮ��̶�Խ����
��2��0.1mol/L��CH3COOH��Һ��ˮϡ�����У��������������������ʵ���������������ʵ�����С��Ũ�ȼ�С�����Լ�����ˮ�����ӻ��������䣬����ĵ���ƽ�ⳣ�����䣻
��3����ͼ��������ˮϡ�͵Ĺ����У�HX��pH�仯�ȽϿ죬˵��HX�����Աȴ���ǿ��
��� �⣺��1���ݵ���ƽ�ⳣ����֪��������ǿ������˳��Ϊ��CH3COOH��H2CO3��HClO��HCO3-�����������Խ����������ӵ�ˮ��̶�Խ����Һ����Խǿ������pH��С��������˳����a��d��c��b���ʴ�Ϊ��a��d��c��b��
��2��0.1mol/L��CH3COOH��Һ��ˮϡ�����У��������������������ʵ�������Ũ�ȼ�С�����Լ�����A��������Ũ�ȼ�С���ʴ���
B����ˮϡ�����У����������ʵ���������������ʵ�����С������$\frac{c��{H}^{+}��}{c��C{H}_{3}COOH��}$ ������ȷ��
C��ˮ�����ӻ��������䣬�ʴ���
D��������Һ��ˮϡ��ʱ���Լ�����������Ũ�ȼ�С����������Ũ����������$\frac{c��O{H}^{-}��}{c��{H}^{+}��}$ ������ȷ��
E������ĵ���ƽ�ⳣ�����䣬�ʴ���
�ʴ�Ϊ��BD��
��3����ͼ��������ˮϡ�͵Ĺ����У�HX��pH�仯�ȽϿ죬˵��HX�����Աȴ���ǿ��HX�ĵ���ƽ�ⳣ���ȴ���ʴ�Ϊ�����ڣ���ˮϡ����ͬ������һԪ���pH�仯Խ������Խǿ������ƽ�ⳣ��Խ��
���� ���⿼��������ʵĵ��룬����ƽ�ⳣ��ȷ������ǿ�����Ӷ�ȷ��ˮ��̶ȣ�ע���ˮϡ��ʱ�Ӹ��������ʵ����仯�������Ѷ��еȣ�

 �㽭֮�ǿ�ʱ�Ż���ҵϵ�д�
�㽭֮�ǿ�ʱ�Ż���ҵϵ�д� ����˼ά�żӿ���ϵ�д�
����˼ά�żӿ���ϵ�д�| ��� | �� | �� | �� |
| �� | CO2 | SO2 | Ca��OH��2 |
| �� | HCl | CO2 | Ca��OH��2 |
| �� | CO2 | SO3 | Ba��NO3��2 |
| �� | NO2 | SO2 | BaCl2 |
| �� | CO2 | NH3 | CaCl2 |
| �� | O2 | NH3 | MgCl2 |
| A�� | �٢ڢ� | B�� | �٢ۢݢ� | C�� | �٢ڢܢ� | D�� | ȫ�� |
| A�� | CH3CH2CH2Cl | B�� | CH3CHBrCH��CH3��CH2Br | ||
| C�� | CH3CHBrCH2CH2Br | D�� | CH3CH2Br |
| A�� | ��ȩ�Ǽ���ȩ�����������ɵ� | |
| B�� | ����һԪȩ��ͨʽ�ɼ�дΪRCOH | |
| C�� | ��������Ӧ����ȩ������������ | |
| D�� | ����һԪ֬��ȩ�ķ�����ɷ���ͨʽCnH2nO |
| A�� | 1mol•L-1��Ca��ClO��2��Һ�к�ClO-��ĿΪ2NA | |
| B�� | ��1molH2SO4��Ũ�����������п��ȫ��Ӧ��ת�Ƶĵ�����Ϊ2NA | |
| C�� | 1mol Mg�ڿ�������ȫȼ������MgO��Mg3N2��ʧȥ2NA������ | |
| D�� | ij�ܱ�����ʢ��0.1molN2��0.3molH2����һ�������·�Ӧ��ת�Ƶĵ�����Ϊ0.6NA |
| A�� | 13C��15N������ͬ�������� | B�� | 13C��C60��ͬһ������ | ||
| C�� | 15N��14N��Ϊͬλ�� | D�� | 15N�ĺ������������������ͬ |
 $\stackrel{R��OH}{��}$
$\stackrel{R��OH}{��}$ +
+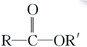
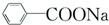 +RCl��
+RCl��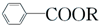 +NaCl
+NaCl ��
��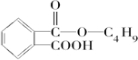 +
+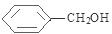 $��_{��}^{Ũ����}$
$��_{��}^{Ũ����}$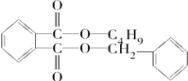 +H2O��
+H2O��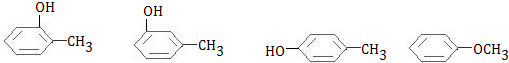 ��
��