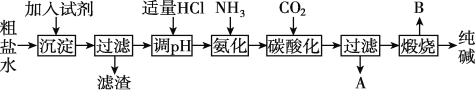
��Ŀ����
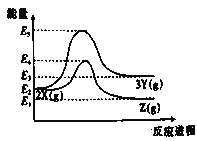
����Ŀ�������£�CuSO4��5H2O(s)��CuSO4(s)����ˮ��Һ֮��ת�����ʱ��ϵ��ͼ��
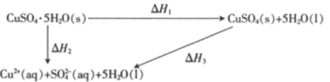
��֪CuSO4��5H2O(s)����ˮ����Һ�¶Ƚ��ͣ�CuSO4(s)����ˮ����Һ�¶����ߡ������й�˵����ȷ����
A.������ͭ��Һ������CuSO4��5H2O(s)�ķ�Ӧ�ʱ��H>0
B.1 mol CuSO4(s)������������1 mol Cu2��(aq)��1 mol SO42��(aq)��������
C.��H2>��H1
D.��H1����H2����H3��
���𰸡�B
��������
A��ͼ����H2��0����֪������ͭ��Һ������CuSO45H2O(s)�ķ�Ӧ�ʱ���H��0����A����
B��ͼ����H3��0����֪1mol CuSO4(s)������������1mol Cu2+(aq)��1mol SO42-(aq)������������B��ȷ��
C����H2��0����H3��0����H1=��H2-��H3������H2����H1����C����
D���ɸ�˹���ɿ�֪��H1=��H2-��H3����D����
�ʴ�ΪB��

��ϰ��ϵ�д�
 ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�
�����Ŀ