��Ŀ����
����Ŀ��������������Ҫ���л��ϳ��м���,�㷺Ӧ���ڻ�ѧ��ҵ��
��1���Ҵ��������Ũ����ļ���˳����___________����ȡ���������ķ�Ӧԭ��Ϊ��__________________________________��
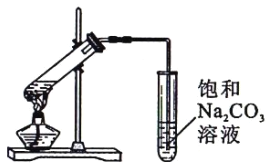
��2��Ϊ֤��Ũ�����ڸ÷�Ӧ������______��________������, Ϊ֤�������ã�ijͬѧ������ͼ��ʾװ�ý����������ĸ�ʵ�顣ʵ�鿪ʼ���þƾ�����3min,�ټ���ʹ֮����3min��ʵ�����������С�Թ�II�ٲ��л���ĺ�ȣ�ʵ���¼����:
ʵ��� �� | �Թ�I�е��Լ� | �Թ�II�е��Լ� | ����л���ĺ��/cm |
A | 2mL�Ҵ���2mL���ᡢ1mL18mo/LŨ���� | 5.0 | |
B | 3mL�Ҵ���2mL���� | ����̼������Һ | 0.1 |
C | 3mL�Ҵ���2mL���ᡢ6mL3mol/L���� | 1.2 | |
D | 3mL�Ҵ���2mL���ᡢ���� | 1.2 |
����D��Ŀ������ʵ��C�����,֤��H+��������Ӧ���д����á�ʵ��D��Ӧ��������������Ũ�ȷֱ���_____mL��_____mol/L��
�ڷ���ʵ��_______(��ʵ����)������,�����Ʋ��Ũ�������ˮ����������������IJ��ʡ�Ũ�������ˮ���ܹ���������������ʵ�ԭ����___________��
��3������̼������Һ���������ܽ�û�з�Ӧ���Ҵ�,��ȥû�з�Ӧ�������___________,��ʵ��װ�������Ե�ȱ�ݣ��������ɵĺ����__________________________��
��4������������������������IJ���,��ʵ�鷢���¶ȹ������������IJ��ʷ������ͣ�һ�����ܵ�ԭ����_______________��
��5�����뱥��̼������Һ�����������ķ�����_________��
���𰸡� �Ҵ���Ũ���������ᣨ���Ҵ���������Ũ������ CH3COOH+CH3CH2OH CH3COOCH2CH3+H2O ���� ��ˮ�� 6 6 AC Ũ��������������Ӧ���ɵ�ˮ���������������Ũ�ȣ�ʹƽ�����������������ķ����ƶ� ���������������ܽ�� �������� �¶ȹ��ߣ��������Ҵ�������ӷ���δ����Ӧ�������˷�Ӧ��ϵ(���¶ȹ��ߣ������˸���Ӧ�����²��ʽ���) ��Һ
CH3COOCH2CH3+H2O ���� ��ˮ�� 6 6 AC Ũ��������������Ӧ���ɵ�ˮ���������������Ũ�ȣ�ʹƽ�����������������ķ����ƶ� ���������������ܽ�� �������� �¶ȹ��ߣ��������Ҵ�������ӷ���δ����Ӧ�������˷�Ӧ��ϵ(���¶ȹ��ߣ������˸���Ӧ�����²��ʽ���) ��Һ
����������1���Ҵ��������Ũ����ļ���˳�����Ҵ���Ũ������������Ҵ���������Ũ�����ȡ���������ķ�Ӧԭ��Ϊ��CH3COOH+CH3CH2OH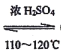 CH3COOCH2CH3+H2O����2����Ӧ��Ũ�������������ˮ�����ã���Ӧ�����ʵ��֤��Ũ�����ڸ÷�Ӧ�����˴��� ����ˮ�����������ټ�Ȼ�Ƕ���ʵ�飬�������ӵ�Ũ��Ӧ����ͬ�ģ����������Ũ����6mol/L�������6mL���ڸ���A��C�����ɵ������������������жϣ�Ũ��������������Ӧ���ɵ�ˮ���������������Ũ�ȣ�ʹƽ�����������������ķ����ƶ�����3������̼������Һ���������ܽ�û�з�Ӧ���Ҵ�,��ȥû�з�Ӧ������ͽ��������������ܽ��,��ʵ��װ�������Ե�ȱ�ݣ��������ɵĺ���Ƿ�����������4������������������������IJ���,��ʵ�鷢���¶ȹ������������IJ��ʷ������ͣ�һ�����ܵ�ԭ�����¶ȹ��ߣ��������Ҵ�������ӷ���δ����Ӧ�������˷�Ӧ��ϵ(���¶ȹ��ߣ������˸���Ӧ�����²��ʽ���)����5������̼������Һ���������������ֲܷ㣬���뱥��̼������Һ�����������ķ����Ƿ�Һ��
CH3COOCH2CH3+H2O����2����Ӧ��Ũ�������������ˮ�����ã���Ӧ�����ʵ��֤��Ũ�����ڸ÷�Ӧ�����˴��� ����ˮ�����������ټ�Ȼ�Ƕ���ʵ�飬�������ӵ�Ũ��Ӧ����ͬ�ģ����������Ũ����6mol/L�������6mL���ڸ���A��C�����ɵ������������������жϣ�Ũ��������������Ӧ���ɵ�ˮ���������������Ũ�ȣ�ʹƽ�����������������ķ����ƶ�����3������̼������Һ���������ܽ�û�з�Ӧ���Ҵ�,��ȥû�з�Ӧ������ͽ��������������ܽ��,��ʵ��װ�������Ե�ȱ�ݣ��������ɵĺ���Ƿ�����������4������������������������IJ���,��ʵ�鷢���¶ȹ������������IJ��ʷ������ͣ�һ�����ܵ�ԭ�����¶ȹ��ߣ��������Ҵ�������ӷ���δ����Ӧ�������˷�Ӧ��ϵ(���¶ȹ��ߣ������˸���Ӧ�����²��ʽ���)����5������̼������Һ���������������ֲܷ㣬���뱥��̼������Һ�����������ķ����Ƿ�Һ��

 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�����Ŀ����һ������SO2(g)��O2(g)�ֱ�ͨ�����Ϊ2L�ĺ����ܱ������У��ڲ�ͬ�¶��½��з�Ӧ��2SO2(g)+ O2(g)![]() 2SO3 ��H<0���õ�����е���������������˵������ȷ����
2SO3 ��H<0���õ�����е���������������˵������ȷ����
ʵ���� | �¶�/�� | ƽ�ⳣ��/mol-1��L | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min | ||
SO2 | O2 | SO2 | O2 | ||||
1 | T1 | K1 | 4 | 2 | x | 0.8 | 6 |
2 | T2 | K2 | 4 | 2 | 0.4 | y | t |
A. T1��T2�Ĺ�ϵ��T1 �� T2
B. x= 1.6��y=0.2 ��t<6
C. K1��K2�Ĺ�ϵ��K2��K1
D. ʵ��1��ǰ6min�ķ�Ӧ������(SO2)=0.2 mol��L-1��min-1
����Ŀ��������ͼװ�ÿ��Խ���ʵ�鲢�ܴﵽʵ��Ŀ�ĵ���

ѡ�� | ʵ��Ŀ�� | X���Լ� | Y���Լ� |
A[] | ��MnO2��Ũ������ȡ���ռ����������Cl2 | ����ʳ��ˮ | Ũ���� |
B | ��Cu��ϡ������ȡ���ռ����������NO | ˮ | Ũ���� |
C | ��֤��ʯ�뱥��ʳ��ˮ��Ӧ���ɵ���������ʲ��ռ� | CuSO4��Һ | KMnO4 |
D | CaCO3��ϡ������ȡ���ռ����������CO2 | ����NaHCO3��Һ | Ũ���� |
A. A B. B C. C D. D
