��Ŀ����
����Ŀ��A��B��CΪ������Ԫ�أ������ڱ���������λ����ͼ��ʾ��A��C��Ԫ�ص�ԭ�Ӻ��������֮�͵���Bԭ�ӵ���������Bԭ�Ӻ�������������������ȡ�
![]()
(1)д��A��B��C��Ԫ������________��________��________��
(2)C��Ԫ�����ڱ��е�λ����____________________��
(3)B��ԭ�ӽṹʾ��ͼΪ________________��C���⻯����B���⻯����ȶ���ǿ��˳��Ϊ________>________(�ѧʽ)��
(4)�Ƚ�A��C��ԭ�Ӱ뾶A________C��д��A����̬�⻯����A������������Ӧˮ���ﷴӦ�Ļ�ѧ����ʽ______________________��
���𰸡���1���� �� ��
��2���ڶ�������A��
��3��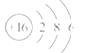 HF H2S
HF H2S
��4��NH3+HNO3=NH4NO3
��������
��A��B��C�����ڱ��е�λ�ÿ�֪��A��C���ڵڶ����ڣ�B���ڵ������ڣ���B��ԭ������Ϊx����AΪx-9��CΪx-7����������x-9+x-7=x����x=16��������Bԭ�Ӻ�������������������ȣ���B��������Ϊ16����S����ôAΪN��CΪF��

����Ŀ��ʵ��������þ��Ϊԭ����ȡMgSO4��7H2O�ֲ�Ʒ�Ĺ������£�
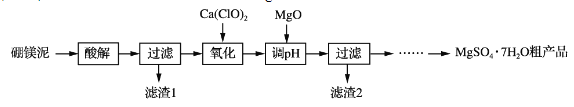
��þ�����Ҫ�ɷ����±���
MgO | SiO2 | FeO��Fe2O3 | CaO | Al2O3 | B2O3 |
30%��40% | 20%��25% | 5%��15% | 2%��3% | 1%��2% | 1%��2% |
(1)����⡱ʱΪ���Mg2���Ľ����ʣ��ɲ��õĴ�ʩ��_____(дһ��)��������˹���̫���ԭ����_____��
(2)�������������У�����H2O2����Ca(ClO)2��������Ӧ�����ӷ���ʽΪ______________��ʵ��δʹ��H2O2����H2O2�ɱ����⣬�����ܵ�ԭ����______________________________________��
(3)����pH��ʱ��MgO������NaOH��Һ��ԭ����________________________��
(4)��ϸ�����Ϣ����MgSO4��7H2O�ֲ�Ʒ(������CaSO4)�ᴿ��ȡMgSO4��7H2O��ʵ�鷽�����£����ֲ�Ʒ����ˮ��_________________________________________________����������Ȼ�ӷ����(ʵ���б���ʹ�õ��Լ��У�����MgSO4��Һ���Ҵ�)���������ε��ܽ��(g/100 gˮ)
�¶ȡ� | 10 | 30 | 40 | 50 |
CaSO4 | 0.19 | 0.21 | 0.21 | 0.20 |
MgSO4��7H2O | 30.9 | 35.5 | 40.8 | 45.6 |