��Ŀ����
��6�֣�1.52gͭþ�Ͻ���ȫ�ܽ���50mL�ܶ�Ϊ1.40g��mL-1����������Ϊ63%��Ũ�����У��õ�NO2��N2O4�Ļ������1120mL(��״��)����Ӧ�����Һ�м���1.0mol��L-1NaOH��Һ������������ȫ������ʱ���õ�2.54g������
��1���úϽ���ͭ��þ�����ʵ���֮���� ��
��2��NO2��N2O4�Ļ�������У�NO2����������� ��
��3���õ�2.54 g����ʱ������NaOH��Һ������� mL��
2:1��80%��640
���������������1����������ȫ������ʱ���õ�2.54g����Ϊ������ͭ��������þ���ʳ�����������������Ϊ2.54g-1.52g=1.02g�������������ʵ���Ϊ1.02g��17g/mol��0.06mol�����ݵ���غ��֪�������ṩ�ĵ������ʵ������������������ʵ�������ͭ��þ�Ͻ���Cu��Mg�����ʵ����ֱ�Ϊxmol��ymol����2x+2y��0.06��64x+24y��1.52�����x=0.02��y=0.01���ʺϽ���ͭ��þ�����ʵ���֮����0.02mol��0.01mol=2��1��
��2��NO2��N2O4�����������ʵ���Ϊ1.12L��22.4L/mol��0.05mol����������������ʵ���Ϊamol�������������������ʵ���Ϊ��0.05-a��mol�����ݵ���ת���غ��֪��a��1+��0.05-a����2��1=0.06�����a=0.04������NO2��N2O4�Ļ�������У�NO2�����������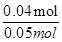 ��100%��80%��
��100%��80%��
��3����Ӧ������Ϊ�����ƣ����ݵ�Ԫ���غ��֪�������Ƶ����ʵ���Ϊ0.05L��14mol/L-0.04mol-��0.05-0.04����2=0.64mol�������������غ��֪n��NaOH��=n��NaNO3��=0.64mol������Ҫ����������Һ�����Ϊ0.64mol��1mol/L��0.64L��640mL��
���㣺����þͭ�Ͻ������ᷴӦ���йؼ���

��֪����ԭ��HSO��3>I����������IO��3>I2���ں�0.3mol NaHSO3����Һ����μ���KIO3��Һ������KIO3������I2�����ʵ����Ĺ�ϵ��������ͼ��ʾ������˵������ȷ����
| A��0��b��ķ�Ӧ�����������ӷ���ʽ��ʾ��3HSO3����IO3��=3SO42����I����3H�� |
| B��a��ʱ����NaHSO3�����ʵ���Ϊ0.12mol |
| C������Һ��I����I2�����ʵ���֮��Ϊ5��2ʱ�������KIO3Ϊ0.18mol |
| D��b��ʱ�Ļ�ԭ���������KI��NaI��b��c��Ļ�ԭ������I2 |
������NO�ķ�Ӧԭ��Ϊ��2CO��2NO=N2��2CO2�йظ÷�Ӧ��˵������ȷ���� ( )
| A����Ӧ��COΪ������ |
| B����Ӧ��NO����ԭ |
| C���ڷ�Ӧ����1 mol N2ʱ��ת�Ƶĵ���Ϊ4 mol |
| D��CO��NO������ɫ�ж����� |
��Fe2+��NO-3��Fe3+��NH+4��H+��H2O���������ֱ�����ͬһ��������ԭ��Ӧ�еķ�Ӧ����������������������ǣ� ��
| A���������뻹ԭ�������ʵ���֮��Ϊ8:1 |
| B����ԭ����ΪNH+4 |
| C������l mol NO-3�μӻ�ԭ��Ӧ����ת��8mol e- |
| D�����Ѹ÷�Ӧ���Ϊԭ��أ�����ӦΪFe2+��e-=Fe3+ |
(10��)Ϊ�˷�ֹǹ֧���⣬����ǹ֧�ĸ����������NaNO2��NaOH�Ļ��Һ�н��л�ѧ����ʹ���������������Fe3O4�����ܵı����㡪����������������̿������л�ѧ����ʽ��ʾ�� �� 3Fe��NaNO2��5NaOH��3Na2FeO2��H2O��NH3��
�� ��Na2FeO2����NaNO2����H2O ������Na2Fe2O4����NH3������NaOH
�� Na2FeO2��Na2Fe2O4��2H2O Fe3O4��4NaOH
Fe3O4��4NaOH
��1����ƽ��ѧ����ʽ�ڡ�
��2��������Ӧ���л�ԭ��Ϊ ������1mol Na2FeO2���ɣ���Ӧ�������� mol����ת�ơ�
��3�������γɡ��������Ĺ��̣�����˵����ȷ���� ��
| A�����������̲��������Ⱦ | B����Ӧ�����ɵ��������������п���ʴ���� |
| C����Ӧ�٢ڢ۾���������ԭ��Ӧ | D����Ӧ�٢��е���������ΪNaNO2 |
���������������������������������������������������������������� ��
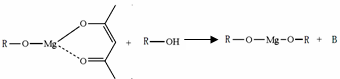
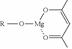 �����ɷ������·�Ӧ��
�����ɷ������·�Ӧ��

 �� ��Ӧ�ٵĻ�ѧ����ʽΪ ��
�� ��Ӧ�ٵĻ�ѧ����ʽΪ ��