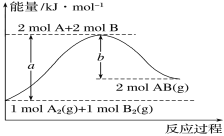
��Ŀ����
����Ŀ����.Ϊ̽������ͭ����(CuSO4��xH2O)���ȷֽ�����ò�����ʵ��װ����ͼ��ʾ������ʵ������Ϊ��A����ɫ������ɰ�ɫ��ĩ�������������ձ�ɺ�ɫ��B�в�����ɫ������D����Һ��ɺ�ɫ��(ϴ��ƿ���Լ�������)

(1)�����Ʋ������ͭ�������շֽ���������_______________________________��
(2)D�еķ�Ӧ���������У�д����һ����Ӧ�����ӷ���ʽ_____________________��
II.�ⶨ����ͭ����(CuSO4��xH2O)�нᾧˮx��ֵ��ʵ��װ�ú������£�ȡ����ͭ����7.23 g����Ӳ���Թ��У���ͨN2�ų���ϵ�ڿ������ƾ���Ƹ��¼��ȳ�֣���A����ɫ�������ձ�ɺ�ɫ��ֹͣ���ȣ��ٴι���N2��װ����ȴ�����¡�(ϴ��ƿ���Լ�������)
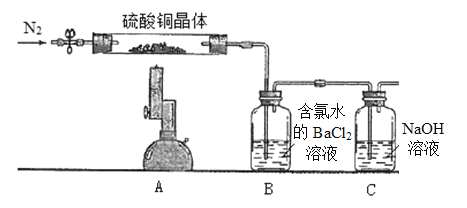
(1)ȡB�а�ɫ����������ϴ�Ӹ�������ù���6.99 g��������ɵ�CuSO4��xH2O��x=__________���ٴι���N2��Ŀ����____________________��
(2)ijͬѧ�����Ҫ�ⶨ�����нᾧˮx��ֵ��Ҳ�ɽ�Bװ����װ��Ũ�����ϴ��ƿ�滻�����ղ�Ũ�������ؼ��ɼ���õ���������۸�ͬѧ�ķ����Ƿ���У�(��������У���˵������)_____________________________
���𰸡�CuO��SO3��SO2��O2��H2O 4Fe2++O2+4H+ = 4Fe3++2H2O 4.5 ʹ�ֽ����������ȫ����ϴ��ƿ���Լ����� �����У�SO3Ҳ���ܽ���Ũ�����У�Ũ��������ز�ֹֻ��ˮ������
��������
I����ʵ���Ŀ����̽������ͭ����(CuSO4��xH2O)���ȷֽ�����ò��A����ɫ������ɰ�ɫ��ĩ�������������ձ�ɺ�ɫ��˵���������ȷֽ������CuO��B������BaCl2��Һ������ɫ��������ֽ��������SO3��D����Һ��죬˵��Fe2+������ΪFe3+���������������������ʣ����������غ㶨�ɿ�֪��������������ΪO2����ֽ��������SO2���ɣ�CuSO4�У�OΪ-2�ۣ����õ�O2����Ҫ�����ϼۣ�����S�Ļ��ϼ�һ�����ͣ�����SO2��������B�е�������Ʒ����Һ��ɫ��E�����������ն�����к����壬��ֹ��Ⱦ������
II������ʵ��I�Ƴ�����ͭ���������SO2��SO3��B�к���Cl2��BaCl2��Cl2��ˮ�п��Խ�SO2����ΪSO42-����CuSO4�е�Sȫ��ת��Ϊ��BaSO4�������ͨ��BaSO4������������x��ֵ��
I����1����������CuSO4���ȷֽ�����IJ�����CuO��SO2��SO3��O2�����ھ�����ԣ����ȷֽ���ﻹ��H2O���ʿ��Ʋ������ͭ�������շֽ���������CuO��SO3��SO2��O2��H2O��
��2��D�У�Fe2+�ȱ�O2����ΪFe3+��Fe3+�ٺ�SCN-�������Ѫ��ɫ���ʣ��漰�����ӷ�ӦΪ��4Fe2++O2+4H+=4Fe3++2H2O��Fe3++3SCN-=Fe(SCN)3��
II����1�����������֪��n(BaSO4)=![]() =0.03mol����n(CuSO4��xH2O)=0.03mol��������0.03mol��(150+18x)g/mol=7.23g�����x=4.5���ٴ�ͨ��N2��Ŀ����ʹ�ֽ����������ȫ����ϴ��ƿ���Լ����գ�
=0.03mol����n(CuSO4��xH2O)=0.03mol��������0.03mol��(150+18x)g/mol=7.23g�����x=4.5���ٴ�ͨ��N2��Ŀ����ʹ�ֽ����������ȫ����ϴ��ƿ���Լ����գ�
��2�������У��ֽ����SO3�����ܽ���Ũ�����У�Ũ��������ز�ֹֻ��ˮ��������