��Ŀ����
20��A��B��C��D��E�����ڱ���ǰ�����ڵ�Ԫ�أ����й����ʻ�ṹ��Ϣ���±���| Ԫ�� | �й����ʻ�ṹ��Ϣ |
| A | �����۵�AԪ�ص��⻯����ͨ��״������һ��Һ�壬����A����������Ϊ88.9% |
| B | Bԭ�ӵõ�һ�����Ӻ�3p���ȫ���� |
| C | Cԭ�ӵ�p����������������̬�⻯������������������ˮ���ﷴӦ����һ�ֳ�������X |
| D | DԪ�ص�����ϼ�����ͻ��ϼ۵Ĵ�����Ϊ�㣬�����������ˮ���������Ϊͬ������ǿ |
| E | EԪ�صĺ˵��������Aԭ�ӵĺ˵������BԪ���⻯��ĺ˵����֮�� |
��2��EԪ��ԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d 64s2��
��3����X��ˮ��Һ�����ԣ�����ԡ������ԡ������ԡ�����
��4��C���ʷ����ЦҼ��ͦм��ĸ�����Ϊ1��2��C���⻯����ͬ��Ԫ�ص��⻯���зе���ַ�������ԭ����NH3���Ӽ���������
��5���ø�����������Һ̬H2Aʱ��һ��H2A�������ͷų�һ�����ӣ�ͬʱ����һ�־��н�ǿ�����Ե������ӣ���д���������ӵĵ���ʽ��
 ��д����������������⻯���ˮ��Һ��Ӧ�����ӷ���ʽ��2H2O++H2S=S��+2H2O+2H+��
��д����������������⻯���ˮ��Һ��Ӧ�����ӷ���ʽ��2H2O++H2S=S��+2H2O+2H+��
���� �����۵�AԪ�ص��⻯����ͨ��״������һ��Һ�壬����A����������Ϊ88.9%����AΪ��Ԫ�أ�Bԭ�ӵõ�һ�����Ӻ�3p���ȫ��������BΪ��Ԫ�أ�Cԭ�ӵ�p����������������̬�⻯������������������ˮ���ﷴӦ����һ�ֳ�������X����CΪ��Ԫ�أ�XΪ����泥�DԪ�ص�����ϼ�����ͻ��ϼ۵Ĵ�����Ϊ�㣬�����������Ϊ���Ӿ��壬��Ϊǰ������Ԫ�أ�����DΪ̼Ԫ�أ�EԪ�صĺ˵��������Aԭ�Ӻˡ�BԪ���⻯��ĺ˵����֮�ͣ���EΪ��Ԫ�أ���A��E�ֱ�ΪO��Cl��N��C��Fe���ݴ˴��⣮
��� �⣺�����۵�AԪ�ص��⻯����ͨ��״������һ��Һ�壬����A����������Ϊ88.9%����AΪ��Ԫ�أ�Bԭ�ӵõ�һ�����Ӻ�3p���ȫ��������BΪ��Ԫ�أ�Cԭ�ӵ�p����������������̬�⻯������������������ˮ���ﷴӦ����һ�ֳ�������X����CΪ��Ԫ�أ�XΪ����泥�DԪ�ص�����ϼ�����ͻ��ϼ۵Ĵ�����Ϊ�㣬�����������Ϊ���Ӿ��壬��Ϊǰ������Ԫ�أ�����DΪ̼Ԫ�أ�EԪ�صĺ˵��������Aԭ�Ӻˡ�BԪ���⻯��ĺ˵����֮�ͣ���EΪ��Ԫ�أ���A��E�ֱ�ΪO��Cl��N��C��Fe��
��1��Ԫ��Y��C��һ����ͬ����Ԫ�أ���YΪPԪ�أ���BΪClԪ�أ�����Ԫ�������ɣ�P�ĵ�һ������С��Cl�ĵ�һ�����ܣ��ʴ�Ϊ������
��2��FeΪ26��Ԫ�أ����������Ų�ʽΪ1s22s22p63s23p63d 64s2���ʴ�Ϊ��1s22s22p63s23p63d 64s2��
��3����XΪNH4NO3����ǿ�������Σ���ˮ��������ԣ��ʴ�Ϊ�����ԣ�
��4��C����ΪN2��N2���Ӻ������������ļ��ͦм��ĸ�����Ϊ1��2�����ڰ����Ӽ�����������ʹ��е���ַ������ʴ�Ϊ��1��2�� NH3���Ӽ���������
��5��H2AΪH2O��H2O�ͷų�һ����������H2O+������Ԫ��Ϊ-1�ۣ������ʽΪ ������ǿ�����ԣ���������H2S�������ӷ���ʽΪ 2H2O++H2S=S��+2H2O+2H+���ʴ�Ϊ��
������ǿ�����ԣ���������H2S�������ӷ���ʽΪ 2H2O++H2S=S��+2H2O+2H+���ʴ�Ϊ�� ��2H2O++H2S=S��+2H2O+2H+��
��2H2O++H2S=S��+2H2O+2H+��
���� ������Ҫ�����˵�һ�����ܡ������Ų�ʽ����ѧ��������ʽ�����ӷ�Ӧ��֪ʶ�㣬�е��Ѷȣ�����ؼ�Ҫ����������е���Ϣ��

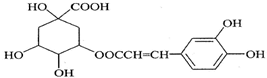
| A�� | ����ʽΪC16H18O9 | |
| B�� | 1 mol��������ˮ��ʱ������8molNaOH | |
| C�� | �뱽����ֱ̼��������ԭ�Ӷ���ͬһƽ���� | |
| D�� | ��Ũ��ˮ���ܷ���ȡ����Ӧ���ܷ����ӳɷ�Ӧ |
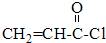 $��_{��Ӧ��}^{CH_{3}CH_{2}OH}$CH2=CH-COOCH2CH3
$��_{��Ӧ��}^{CH_{3}CH_{2}OH}$CH2=CH-COOCH2CH3 ����ע����Ӧ������
����ע����Ӧ������ ��CH2=CH-O-CH2-O-CH=CH2��
��CH2=CH-O-CH2-O-CH=CH2��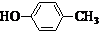 Ҳ���뻯����������Ʒ�Ӧ�۵ķ�Ӧ����õ��IJ���Ľṹ��ʽΪ
Ҳ���뻯����������Ʒ�Ӧ�۵ķ�Ӧ����õ��IJ���Ľṹ��ʽΪ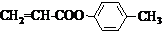 ��
��
 ͨ������·�߿ɺϳɣ���
ͨ������·�߿ɺϳɣ��� $��_{��}^{ŨH_{2}SO_{4}}$C3H4O2����$\stackrel{C_{2}H_{5}OH/H+}{��}$C5H8O2$��_{һ������}^{HBr}$����
$��_{��}^{ŨH_{2}SO_{4}}$C3H4O2����$\stackrel{C_{2}H_{5}OH/H+}{��}$C5H8O2$��_{һ������}^{HBr}$���� ��
�� ����Ӧ������������Ӧ����ȡ����Ӧ����
����Ӧ������������Ӧ����ȡ����Ӧ���� ��
��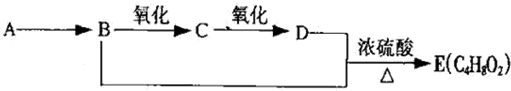
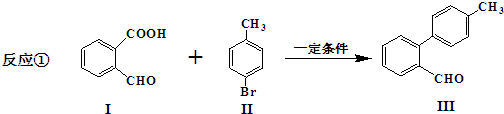
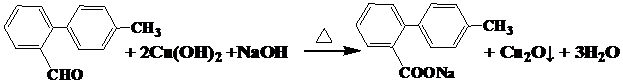 ��
��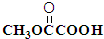 ��
��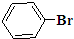 Ҳ���Է������Ʒ�Ӧ�ٵķ�Ӧ���л�����Ľṹ��ʽΪ��
Ҳ���Է������Ʒ�Ӧ�ٵķ�Ӧ���л�����Ľṹ��ʽΪ��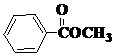 ��
��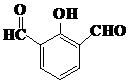 ��
��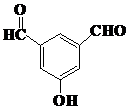 ��
��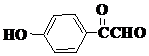 ���������֣�����Ҫ������FeCl3��Һ������ɫ��Ӧ���ڱ�����һ��ȡ��������2�֣�
���������֣�����Ҫ������FeCl3��Һ������ɫ��Ӧ���ڱ�����һ��ȡ��������2�֣�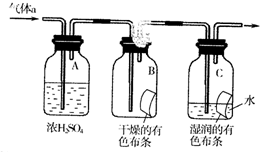 ������һ�ֻ�ѧ���ʻ��õķǽ������ʣ���ҵ��
������һ�ֻ�ѧ���ʻ��õķǽ������ʣ���ҵ��