题目内容
12.已知:乙醛在一定条件下可被氧气氧化为乙酸.A是石油裂解的主要产物之一,其产量常用于衡量一个国家石油化工发展水平的标志.下列是有机物之间的转化关系: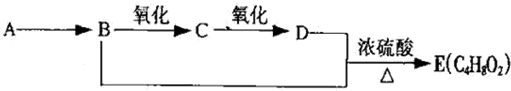
(1)A的结构简式为CH2=CH2,可以作为水果的催熟剂;
(2)B+D→E的反应类型为酯化反应;
(3)写出B→C和B+D→E的两个化学反应方程式:
B→C:2CH3CH2OH+O2→Cu△→Cu△2CH3CHO+2H2O,
B+D→E:CH3CH2OH+CH3COOH?浓硫酸△CH3COOCH2CH3+H2O;
(4)除去E中所混有少量的D杂质,所需试剂的名称是:饱和碳酸钠溶液.
分析 A是石油裂解主要产物之一,其产量可以用来衡量一个国家的石油化工水平,A为C2H4,B能发生连续氧化性生成D,则B为醇、C为醛、D为羧酸、E为酯.故乙烯与水发生加成反应生成B,B为CH3CH2OH,乙醇在Cu或Ag作催化剂条件下发生氧化反应CH3CHO,C为CH3CHO,CH3CHO可进一步氧化物CH3COOH,D为CH3COOH,CH3CH2OH和CH3COOH在浓硫酸作用下反应生成乙酸乙酯,故E为CH3COOCH2CH3,据此解答.
解答 解:A是石油裂解主要产物之一,其产量可以用来衡量一个国家的石油化工水平,A为C2H4,B能发生连续氧化性生成D,则B为醇、C为醛、D为羧酸、E为酯.故乙烯与水发生加成反应生成B,B为CH3CH2OH,乙醇在Cu或Ag作催化剂条件下发生氧化反应CH3CHO,C为CH3CHO,CH3CHO可进一步氧化物CH3COOH,D为CH3COOH,CH3CH2OH和CH3COOH在浓硫酸作用下反应生成乙酸乙酯,故E为CH3COOCH2CH3,
(1)由上述分析可知,A的结构简式为CH2=CH2,可以作为水果的催熟剂,
故答案为:CH2=CH2;催熟剂;
(2)反应B+D→E是乙醇与乙酸在浓硫酸、加热条件下生成乙酸乙酯,属于酯化反应,
故答案为:酯化反应;
(3)B→C的反应方程式为:2CH3CH2OH+O2→Cu△2CH3CHO+2H2O,
B+D→E反应方程式为:CH3CH2OH+CH3COOH ?浓硫酸△CH3COOCH2CH3+H2O;
故答案为:2CH3CH2OH+O2 →Cu△2CH3CHO+2H2O;CH3CH2OH+CH3COOH ?浓硫酸△CH3COOCH2CH3+H2O;
(4)除去乙酸乙酯中所混有少量的乙酸杂质,具体操作为:把E加入分液漏斗中,再加入饱和碳酸钠溶液,充分振荡、静置、分层,然后分液,取上层液体即可,
故答案为:饱和碳酸钠溶液.
点评 本题考查有机物推断、物质分离提纯、烯与醇、醛、羧酸之间的转化关系等,难度不大,注意基础知识的理解掌握.

 开心练习课课练与单元检测系列答案
开心练习课课练与单元检测系列答案 开心试卷期末冲刺100分系列答案
开心试卷期末冲刺100分系列答案| A. | 0.1mol/L pH=9的NaNO2溶液中C(Na+)>C(NO2-)>C(OH-)>C(H+) | |
| B. | 0.1mol/LNa2S溶液中:2C(Na+)=C(S2-)+C(HS-)+C(H2S) | |
| C. | 等PH的氨水、NaOH溶液、Ba(OH)2溶液中:C(NH4+)=C(Na+)=C(Ba2+) | |
| D. | 向NH4HCO3溶液中滴加NaOH溶液至pH=7:C(NH4+)+C(Na+)=C(HCO32-)+C(CO32-) |
| A. | 矿石粉碎的目的是使原料充分利用,并增大接触面使反应速率加快 | |
| B. | 接触室中采用常压的主要原因是常压下SO2的转化率已经很高 | |
| C. | 沸腾炉中出来的混合气需要洗涤,目的是防止催化剂中毒 | |
| D. | 接触室采用450℃的温度是使催化剂活性最佳,提高平衡混和气中SO3的含量 |
| 元素 | 有关性质或结构信息 |
| A | 负二价的A元素的氢化物在通常状况下是一种液体,其中A的质量分数为88.9% |
| B | B原子得到一个电子后3p轨道全充满 |
| C | C原子的p轨道半充满,它的气态氢化物能与其最高价氧化物的水化物反应生成一种常见的盐X |
| D | D元素的最高化合价与最低化合价的代数和为零,其最高价氧化物水化物的酸性为同主族最强 |
| E | E元素的核电荷数等于A原子的核电荷数和B元素氢化物的核电荷数之和 |
(2)E元素原子的核外电子排布式为1s22s22p63s23p63d 64s2;
(3)盐X的水溶液呈酸性(填“酸性”“碱性”或“中性”);
(4)C单质分子中σ键和π键的个数比为1:2,C的氢化物在同族元素的氢化物中沸点出现反常,其原因是NH3分子间存在氢键;
(5)用高能射线照射液态H2A时,一个H2A分子能释放出一个电子,同时产生一种具有较强氧化性的阳离子,试写出该阳离子的电子式:
 ,写出该阳离子与硫的氢化物的水溶液反应的离子方程式:2H2O++H2S=S↓+2H2O+2H+.
,写出该阳离子与硫的氢化物的水溶液反应的离子方程式:2H2O++H2S=S↓+2H2O+2H+. (1)提出假设
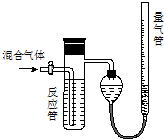
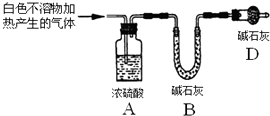
实验I:用砂纸擦去镁条表面氧化膜,将其放入盛有适量滴有酚酞的饱和碳酸氢钠溶液的试管中,迅速反应,产生大量气泡和白色不溶物,溶液由浅红变红.
该同学对反应中产生的白色不溶物做出如下猜测:
猜测1:白色不溶物可能为Mg(OH)2 .
猜测2:白色不溶物可能为MgCO3.
猜测3:白色不溶物可能是碱式碳酸镁[xMgCO3•yMg(OH)2].
(2)设计定性实验确定产物并验证猜测:
| 实验序号 | 实验 | 实验现象 | 结论 |
| 实验Ⅱ | 将实验I中收集到的气体点燃 | 能安静燃烧、产生淡蓝色火焰 | 气体成分为 氢气 |
| 实验Ⅲ | 取实验I中的白色不溶物,洗涤, 加入足量稀盐酸 | 产生气泡沉淀全部溶解 | 白色不溶物可能含有MgCO3 |
| 实验Ⅳ | 取实验I中的澄清液,向其中加 入少量CaCl2稀溶液 | 产生白色沉淀 | 溶液中存在④ CO32-离子 |
称取实验Ⅰ中所得干燥、纯净的白色不溶物22.6g,充分加热至不再产生气体为止,并使分解产生的气体全部进入装置A和B中.实验前后装置A增重1.8g,装置B增重8.8g,试确定白色不溶物的化学式Mg(OH)2(CO3)2或2MgCO3•Mg(OH)2 .
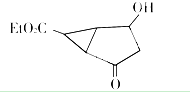
Et代表的基团为-CH2CH3
该有机分子的核磁共振波谱图如下(单位是ppm).
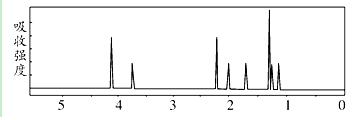
下列关于该有机物的叙述正确的是( )
| A. | 该有机物不同化学环境的氢原子有8种 | |
| B. | 该有机物属于芳香族化合物 | |
| C. | 该有机物分子式为C9H14 O4 | |
| D. | 1 mol该有机物完全燃烧可以产生7 mol水 |
| A. | Na+、K+、SO42-、MnO4- | B. | Ca2+、NH4+、Cl-、NO3- | ||
| C. | Mg2+、K+、HCO3-、Cl- | D. | Na+、K+、SO32-、SO42- |