��Ŀ����
16����������������߲࣬�ԣ�ͼA������ȡ����ʱ����Ϊ߲����߲�����ĸ���ԭ������������ӽ������Ҷ���أ�ͼB���ȶ������ʣ���ش�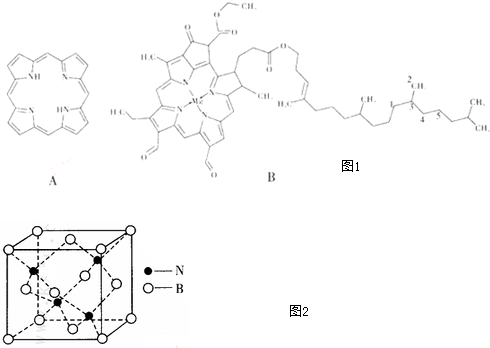
��1��߲����Nԭ�Ӳ��õĹ���ӻ���ʽ��sp3��sp2
��2�������й�Ҷ���ط��ӵ�˵����ȷ����BD����ѡ����ţ���
A��ͼ1��1-5��C����������������
B�������д�����λ��
C��ͼ1��1��2��3��4��C����
D��N�ĵ�һ�����ܴ���O
��3��߲����Fe2+�ϼ����γ�Ѫ���أ�Fe2+�ĵ����Ų�ʽΪ1s22s22p63s23p63d6
��4���軯�⣨HCN����һ�ֺ����綾�����������ЦҼ���м��ĸ�����Ϊ1��1���ɷ��ӽṹ�Ʋ⣬�軯���ף���ס����ס�������ˮ��ԭ����HCN��ˮ���Ǽ��Է��ӣ�������������ԭ��֪��HCN������ˮ���軯���������������CN-��ʹ��Ѹ���ж�����ٳ�����CN-�ĵȵ�����N2��CO��
��5��N��B�ܹ��γ�һ��Ӳ�Ƚӽ����ʯ�����ʣ��侧��ṹ��ͼ2�����侧���߳�Ϊapm�������ܶ�Ϊ$\frac{\frac{25}{{N}_{A}}��4}{��a��1{0}^{-10}��^{3}}$g��cm-3��ֻ����ʽ����
���� ��1�����ݼ۲���ӶԻ�������ȷ���ӻ���ʽ��
��2��A������̼ԭ�������ĸ���ͬ��ԭ�ӻ�ԭ���ţ�
B�����йµ��ӶԺͺ��пչ����ԭ��֮�������λ����
C���ṹ���顢��ϩ�ṹ�ж��Ƿ��棻
D��ͬһ����Ԫ�أ�Ԫ�صĵ�һ����������ԭ��������������������ƣ�����IIA�塢��VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ�
��3��Fe��26��Ԫ�أ���ԭ�Ӻ�����26�����ӣ�ʧȥ�����������������������ӣ����ݹ���ԭ����д���������Ų�ʽ��
��4��HCN�ĽṹʽΪH-C��N�����۵���Ϊ�Ҽ������������к���һ���Ҽ������м������Է��ӵ����ʼ������ڼ��Է��ӵ��ܼ���ԭ�Ӹ�����ȡ��۵�������ȵ�����Ϊ�ȵ����壻
��5���þ�����Nԭ�Ӹ���Ϊ4��Bԭ�Ӹ���=8��$\frac{1}{8}$+6��$\frac{1}{2}$=4���仯ѧʽΪBN���ⳤΪa��10-10cm���������=��a��10-10cm��3�������ܶ�=$\frac{m}{V}$��
��� �⣺��1���۲���ӶԸ�����4��Nԭ�Ӳ���sp3�ӻ����۲���ӶԸ�����3��N����sp2�ӻ���
�ʴ�Ϊ��sp3��sp2��
��2��A������̼ԭ�������ĸ���ͬ��ԭ�ӻ�ԭ���ţ��⼸��̼ԭ��ֻ��3��̼ԭ��������̼ԭ�ӣ��ʴ���
B�����йµ��ӶԺͺ��пչ����ԭ��֮�������λ����Nԭ�Ӻ��йµ��Ӷԡ�����ԭ�Ӻ��пչ�������Ժ�����λ��������ȷ��
C���ṹ���顢��ϩ�ṹ֪���⼸��̼ԭ��֮�䲻�ܹ��棬�ʴ���
D��ͬһ����Ԫ�أ�Ԫ�صĵ�һ����������ԭ��������������������ƣ�����IIA�塢��VA��Ԫ�ص�һ�����ܴ���������Ԫ�أ����Ե縺��N��O������ȷ��
��ѡBD��
��3��Fe��26��Ԫ�أ���ԭ�Ӻ�����26�����ӣ�ʧȥ�����������������������ӣ����ݹ���ԭ����д���������Ų�ʽΪ1s22s22p63s23p63d6���ʴ�Ϊ��1s22s22p63s23p63d6��
��4��HCN�ĽṹʽΪH-C��N�����۵���Ϊ�Ҽ������������к���һ���Ҽ������м����÷����к��������Ҽ������м������Զ���֮��Ϊ1��1��
���Է��ӵ����ʼ������ڼ��Է��ӵ��ܼ���HCN�Ǽ��Է��ӡ�ˮ�Ǽ��Է��ӣ�����HCN������ˮ��
ԭ�Ӹ�����ȡ��۵�������ȵ�����Ϊ�ȵ����壬CN-�ĵȵ�������N2��CO�ȣ�
�ʴ�Ϊ��1��1���ף�HCN��ˮ���Ǽ��Է��ӣ�������������ԭ��֪��HCN������ˮ��N2��CO��
��5���þ�����Nԭ�Ӹ���Ϊ4��Bԭ�Ӹ���=8��$\frac{1}{8}$+6��$\frac{1}{2}$=4���仯ѧʽΪBN���ⳤΪa��10-10cm���������=��a��10-10cm��3�������ܶ�=$\frac{m}{V}$=$\frac{\frac{M}{{N}_{A}}��4}{V}$=$\frac{\frac{25}{{N}_{A}}��4}{��a��1{0}^{-10}��^{3}}$g��cm-3��
�ʴ�Ϊ��$\frac{\frac{25}{{N}_{A}}��4}{��a��1{0}^{-10}��^{3}}$��
���� ���⿼�龧�����㡢��������ԭ���������Ų���Ԫ�������ɼ���ѧ����֪ʶ�㣬��Щ���Ǹ�Ƶ���㣬�����ü۲���ӶԻ������ۡ�����ԭ����֪ʶ���������ѵ��Ǿ������㣬֪���ܶȹ�ʽ�и�����ĸ�ĺ��壬��Ŀ�Ѷ��еȣ�

 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д� ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�| A�� | 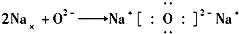 | B�� | 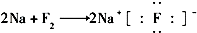 | ||
| C�� | 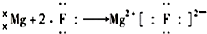 | D�� | 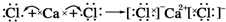 |
| A�� | NH4+��Cl-��Na+��SO42- | B�� | Na+��Ba2+��OH-��SO42- | ||
| C�� | Ca2+��H+��S2-��ClO- | D�� | H+��Cl-��Fe2+��NO3- |
| A�� | KMnO4��Һ�������ữ������H2O2��2MnO4-+6H++5H2O2=2Mn2++5O2��+8H2O | |
| B�� | Fe2��SO4��3��Ba��OH��2��Һ��ϣ�Fe3++SO42-+Ba2++3OH-=BaSO4��+Fe��OH��3�� | |
| C�� | ����������ʵ���Ũ�ȵ�AlCl3��Ba��OH��2��HCl��Һ��ϣ�3H++Al3++6OH-=Al��OH��3��+3H2O | |
| D�� | ˮ������ϡ�����ϣ�SiO32-+2H+=H2SiO3�� |
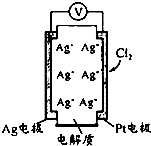 ������ͼ��ʾԭ��ؿɲ���������Cl2���������е������Ag+���������ƶ��Ĺ������ʣ����з�������ȷ���ǣ�������
������ͼ��ʾԭ��ؿɲ���������Cl2���������е������Ag+���������ƶ��Ĺ������ʣ����з�������ȷ���ǣ�������| A�� | ���Ӿ����·����Pt�缫 | |
| B�� | ������Ӧ��Cl2+2e-+2Ag+=2AgCl | |
| C�� | ��ع���ʱ���������Ag+��Ŀ���� | |
| D�� | ������c��Cl2��Խ��Ag����������Խ�� |
| A�� | HX | B�� | H2X | C�� | XH4 | D�� | XH3 |
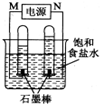 CO��H2��Ϊ��Ҫ��ȼ�Ϻͻ���ԭ�ϣ�����ʮ�ֹ㷺��Ӧ�ã�
CO��H2��Ϊ��Ҫ��ȼ�Ϻͻ���ԭ�ϣ�����ʮ�ֹ㷺��Ӧ�ã�