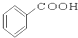
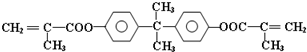
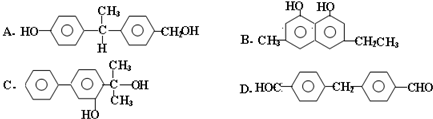
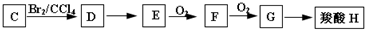��Ŀ����
14��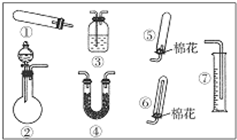 ijͬѧ����ͼװ�ã��̶����������������ԣ������йذ�����ȡ��ʵ��̽�����ش��������⣺
ijͬѧ����ͼװ�ã��̶����������������ԣ������йذ�����ȡ��ʵ��̽�����ش��������⣺��1������װ�â���ȡNH3���䷴Ӧ�Ļ�ѧ����ʽ2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
��Ҫ�ⶨ���ɵ�NH3������������ѡ���װ���Ǣۢߣ���װ����ţ���װ������ʢ�Լ�Ӧ���е������ǰ�����Һ�岻�ܷ�Ӧ�������������ڸ�Һ�塢��Һ�岻�ܻӷ���
��2������װ�â���ȡ���ռ������NH3����ƿ��װ���Լ���CaO��NaOH���ʯ�ң�����Һ©����װ���Լ���Ũ��ˮ����ŨNH4Cl��Һ�����ռ�װ��Ӧѡ��ޣ���װ����ţ���֤���������ռ����IJ�������ʪ��ĺ�ɫʯ����ֽ�����ܿڣ�����ֽ������֤�����ռ�����
��3����д�����Ĵ������Ļ�ѧ����ʽ4NH3+5O2$\frac{\underline{\;����\;}}{��}$4NO+6H2O
��д��ͭ��ϡ���ᷴӦ�����ӷ���ʽ3Cu+2NO3-+8H+=2NO��+3Cu2++4H2O
��4����γ�ȥһ�������е����ʶ����������û�ѧ����ʽ˵����3NO2+H2O=2HNO3+NO��
���� ��1��װ�â��Ʊ����������Ϊ�������������ȣ�
�ⶨ��������������Բ�����Һ��ķ����������Ұ�����Һ�岻�ܷ�Ӧ�������������ڸ�Һ�塢��Һ�岻�ܻӷ���
��2��Ũ��ˮ�ӷ���Ũ��ˮ���뵽��ʯ�ң���ˮ��Ӧ�����������ƣ��ų��������ȣ��ܹ��ٽ���ˮ�Ļӷ�������������������������Ȼ�識����Ʊ�������
�����ܶ�С�ڿ�����Ӧѡ�������������ռ�������Ϊ�������壬����ʪ��ĺ�ɫʯ����ֽ�������ݴ˿��Լ��鰱���Ƿ��ռ�����
��3�������������ڴ��������¼�������һ��������ˮ��ͭ��ϡ���ᷴӦ��������ͭ��һ��������ˮ��
��4��һ������������ˮ��������������ˮ��ˮ��Ӧ���������һ��������
��� �⣺��1������װ�â���ȡNH3����ʵ�������Ȼ�狀����������ڼ�����������ȡ��������Ӧ����ʽ��2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O��
Ҫ�ⶨ��������������Բ�����Һ��ķ����������Ұ�����Һ�岻�ܷ�Ӧ�������������ڸ�Һ�塢��Һ�岻�ܻӷ���
�ʴ�Ϊ��2NH4Cl+Ca��OH��2$\frac{\underline{\;\;��\;\;}}{\;}$CaCl2+2NH3��+2H2O���ۢߣ�������Һ�岻�ܷ�Ӧ�������������ڸ�Һ�塢��Һ�岻�ܻӷ���
��2��Ũ��ˮ�ӷ���Ũ��ˮ���뵽��ʯ�ң���ˮ��Ӧ�����������ƣ��ų��������ȣ��ܹ��ٽ���ˮ�Ļӷ������������Ʊ������������ܶ�С�ڿ�����Ӧѡ�������������ռ�������Ӧѡ��ޣ�����Ϊ�������壬����ʪ��ĺ�ɫʯ����ֽ�������ݴ˿��Լ��鰱���Ƿ��ռ�����
�ʴ�Ϊ����ʯ�ң�Ũ��ˮ���ޣ���ʪ��ĺ�ɫʯ����ֽ�����ܿڣ�����ֽ������֤�����ռ�����
��3����������������һ��������ˮ������ʽ��4NH3+5O2$\frac{\underline{\;����\;}}{��}$4NO+6H2O��ͭ��ϡ���ᷴӦ��������ͭ��һ��������ˮ����Ӧ�����ӷ���ʽ��3Cu+2NO3-+8H+=2NO��+3Cu2++4H2O��
�ʴ�Ϊ��4NH3+5O2$\frac{\underline{\;����\;}}{��}$4NO+6H2O��3Cu+2NO3-+8H+=2NO��+3Cu2++4H2O��
��4��һ������������ˮ��������������ˮ��ˮ��Ӧ���������һ�����������������ͨ��ʢ��ˮ��ϴ��ƿ���ɳ�ȥһ�������еĶ�������������ʽ3NO2+H2O=2HNO3+NO���ʴ�Ϊ��3NO2+H2O=2HNO3+NO��
���� ���⿼���˰�����ʵ�����Ʊ������ʵļ��飬��ȷ�Ʊ�ԭ����װ��ѡ���ǽ���ؼ���ע�ⰱ�����ռ�������ķ�������Ŀ�ѶȲ���

| A�� | ������������ʱ�������¶ȣ���ѧ��Ӧ���ʼӿ� | |
| B�� | ������������ʱ������Ũ�ȣ���ѧ��Ӧ���ʼӿ� | |
| C�� | ��ѧ��Ӧ�ﵽ��Ӧ��ʱ����Ӧ���Ũ�����������Ũ����� | |
| D�� | ��ѧ��Ӧ�����Dz����Ըı�� |
| A�� | �������м���Ũ�������ַ�������˵��Ũ���������ˮ�� | |
| B�� | ��ij��Һ�м����Ȼ�����Һ��ϡ���ᣬ���ɰ�ɫ��������ԭ��Һһ������SO42- | |
| C�� | �����£���ͭ����Ũ�����������Ա仯��˵��ͭ�����Ũ�����жۻ� | |
| D�� | Ũ�����ڹ����±�ƣ�֤������ȶ����Ҳ����к���ɫ���������Ũ���� |
| A�� | C60����ʯһ������ԭ�Ӿ��� | |
| B�� | �ɱ������ƻ��˹��ۼ� | |
| C�� | ���ۻ�������һ���������Ӽ� | |
| D�� | �Ȼ�������ˮ�ܵ����H+��Cl-�������Ȼ��������ӻ����� |
| A�� | ��Ba��OH��2��Һ�еμ�NH4HSO4��Һ���պó�����ȫ��Ba2++2OH-+NH4++H++SO42-=BaSO4��+NH3•H2O+H2O | |
| B�� | H218O��Ͷ��Na2O2���壺2H218O+2O22-=4OH-+18O2 | |
| C�� | 0.1mol/L CuCl2��Һ�м���0.1mol/L NaHS��Һ��Cu2++2HS-+2H2O=Cu��OH��2��+2H2S�� | |
| D�� | ��ʯī���缫���AlCl3��Һ��2Cl-+2H2O $\frac{\underline{\;���\;}}{\;}$ Cl2��+H2��+2OH- |
| A�� | ���õ����еĽ������� | B�� | ��ֹ���������Ⱦ | ||
| C�� | �ϵ�������������������ʹ�� | D�� | �������зǽ������� |
| A�� | ��ͬ���ʵ�����������ȫȼ�գ����ɵ�CO2Խ�࣬˵�����е�̼����������Խ�� | |
| B�� | ��ͬ������������ȫȼ�գ����ĵ�O2Խ�࣬˵�����е������������Խ�� | |
| C�� | ������Ӧʵ����Թ��ڱڸ����������ð�ˮ��ϴ | |
| D�� | ��������CF2Cl2���������NOx��������ƻ����������㣬�Ӷ����¡�����ЧӦ�� |
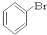
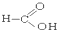
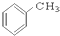
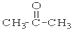 ��CH3CH2Br ��
��CH3CH2Br ��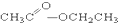 ��
��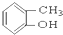 ��
�� ��
��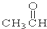 ��
�� ��
�� ��
��