��Ŀ����
5����ͼ��Ԫ�����ڱ���һ����
��1��д��Ԫ�آ�Ļ�̬ԭ�ӵĵ����Ų���ʽ[Ar]3d64s2����Χ�����Ų�ͼ3d64s2��ָ���������ڱ��е�λ�õ������ڵڢ����壮���Ӹֹ�ʱ�������â��ijЩ��������ߵĵ����ڸ����·�����Ӧ����д������һ����Ӧ�Ļ�ѧ����ʽ2Al+Fe2O3$\frac{\underline{\;����\;}}{\;}$Al2O3+2Fe��
��2���٢ۢ�����Ԫ�ؿ����γɶ����л���������ӣ��������ԭ�������ٵ�һ��������װ��ʱ�γɵ���Ҫ������Ⱦ���д�����ĵ���ʽ
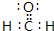 ���Ʋ�÷��ӵĿռ乹��Ϊƽ�������Σ�
���Ʋ�÷��ӵĿռ乹��Ϊƽ�������Σ���3���ۢܢݢޢ�����Ԫ�ض�������Ԫ�آ��γɻ���������۵���ߵ���NaH��д������Ļ�ѧʽ����������¶Ƚӽ�373Kʱ������M=m/n�ⶨ�ݵ���̬�⻯�����Է���������������ֲⶨ������DZ�����ֵ�ߣ���ԭ����ˮ���Ӽ�������ˮ��������Ӽ��γɵ��
��4��ijЩ��ͬ��Ԫ�ص�����Ҳ��һ���������ԣ���ͼ��Ԫ�آ���Ԫ�آڵ��������������Ƶ����ʣ���д��Ԫ�آڵ�����������NaOH��Һ��Ӧ�Ļ�ѧ����ʽBe��OH��2+2NaOH=Na2BeO2+2H2O��
���� ��Ԫ�������ڱ��е�λ�ÿ�֪����ΪH����ΪBe����ΪC����ΪN����ΪO����ΪNa����ΪAl����ΪCl����ΪCr����ΪFe��
��1��Fe��ԭ������Ϊ26���۵���Ϊ3d64s2��λ�ڵ������ڵڢ����壬���ijЩ��������ߵĵ����ڸ����·������ȷ�Ӧ��
��2���٢ۢ�����Ԫ���γ��л��������ԭ������������������װ��ʱ�γɵ���Ҫ������Ⱦ�������Ϊ��ȩ��
��3���ۢܢݢޢ�����Ԫ�ض�������Ԫ�آ��γɻ����ֻ���������γ��⻯�����Ӿ��壬�������Ƿ��Ӿ��壬�����⻯�Ƶ��۵���ߣ�ˮ���Ӽ��������γɵ��
��4��Ԫ�آ���Ԫ�آڵ��������������Ƶ����ʣ�Ԫ�آڵ�����������NaOH��Һ��Ӧ����Na2BeO2��ˮ��
��� �⣺��Ԫ�������ڱ��е�λ�ÿ�֪����ΪH����ΪBe����ΪC����ΪN����ΪO����ΪNa����ΪAl����ΪCl����ΪCr����ΪFe��
��1��Fe��ԭ������Ϊ26����̬ԭ�ӵĵ����Ų���ʽ[Ar]3d64s2���۵���Ϊ3d64s2��λ�ڵ������ڵڢ����壬���ijЩ��������ߵĵ����ڸ����·������ȷ�Ӧ����2Al+Fe2O3$\frac{\underline{\;����\;}}{\;}$Al2O3+2Fe��
�ʴ�Ϊ��[Ar]3d64s2��3d64s2���������ڵڢ����壻2Al+Fe2O3$\frac{\underline{\;����\;}}{\;}$Al2O3+2Fe��
��2���٢ۢ�����Ԫ���γ��л��������ԭ������������������װ��ʱ�γɵ���Ҫ������Ⱦ�������Ϊ��ȩ������ʽΪ��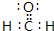 ��������Cԭ�ӳ�2��C-H����1��C=O����Cԭ�Ӽ۲���Ӷ���Ϊ2+1=3�������µ��Ӷԣ�Ϊƽ�������Σ�
��������Cԭ�ӳ�2��C-H����1��C=O����Cԭ�Ӽ۲���Ӷ���Ϊ2+1=3�������µ��Ӷԣ�Ϊƽ�������Σ�
�ʴ�Ϊ��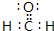 ��ƽ�������Σ�
��ƽ�������Σ�
��3���ۢܢݢޢ�����Ԫ�ض�������Ԫ�آ��γɻ����ֻ���������γ��⻯�����Ӿ��壬�۵���ߣ�ˮ���Ӽ��������γɵ�����Բⶨ������DZ�����ֵ�ߣ��ʴ�Ϊ��NaH��ˮ���Ӽ�������ˮ��������Ӽ��γɵ��
��4��Ԫ�آ���Ԫ�آڵ��������������Ƶ����ʣ�Ԫ�آڵ�����������NaOH��Һ��Ӧ����Na2BeO2��ˮ���÷�ӦΪBe��OH��2+2NaOH=Na2BeO2+2H2O��
�ʴ�Ϊ��Be��OH��2+2NaOH=Na2BeO2+2H2O��
���� ���⿼��Ԫ�����ڱ���Ӧ�ã�����Ԫ�������ڱ��е�λ�á�ԭ�ӽṹ�����ʡ�Ԫ�ػ�����֪ʶΪ���Ĺؼ�������Ԫ���ƶϼ���ѧ����Ŀ��飬ע�⣨4�������ʵ����ƣ���Ŀ�ѶȲ���

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д��������/% ѹǿ/MPa �¶�/�� | 1.0 | 2.0 | 3.0 |
| 810 | 54.0 | a | b |
| 915 | c | 75.0 | d |
| 1 000 | e | f | 83.0 |
| A�� | a��54.0 | B�� | b��f | ||
| C�� | 915�棬2.0 MPaʱE��ת����Ϊ60% | D�� | K��1000�棩��K��810�棩 |
| A�� | 32g O2������ԭ����ĿΪNA | |
| B�� | 0.5mol H2O�����е�ԭ����Ϊ0.5NA | |
| C�� | 1mol H2���е�ԭ����ĿΪ2NA | |
| D�� | 0.5NA���������ӵ����ʵ�����0.5mol |
| A�� | SO2��CO2�������� | |
| B�� | SO2��ʹƷ����Һ��ɫ��CO2���� | |
| C�� | SO2��CO2���ܸ�ʯ��ˮ��Ӧ���ɰ�ɫ���� | |
| D�� | SO2��CO2���������������л�ԭ�� |
| A�� | ���� | B�� | �Ҵ� | ||
| C�� | ��ȩ | D�� | �״��ͱ������Ļ���� |
| A�� | �÷�Ӧ���ʱ�Ϊ��ֵ | |
| B�� | ����������������ӵ�λ����ڵĻ�ٷ����� | |
| C�� | ������䣬���뺤����ѹǿ����ѧ��Ӧ�������� | |
| D�� | �����¶ȣ�K��С |
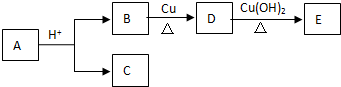
| A�� | ��������ת����ϵ���л���A�Ľṹ��8�� | |
| B�� | C��Eһ��Ϊͬϵ�� | |
| C�� | 1molB��ȫת��ΪDת��2mol���� | |
| D�� | D��E����������Ӧ |
| A�� | �� | B�� | �� | C�� | �Ȼ�þ | D�� | �������� |