��Ŀ����
��14�֣�����A��B��C��D��E��F��G��H���ֶ���������Ԫ�أ�ԭ����������������֪A��E��D��G�ֱ�ͬ���壻E��F��G��Hͬ���ڣ�A�ֱ���C��D���γɺ���10�����ӵĹ��ۻ�����M��N��B������������������Ӳ�����2����D�ǵؿ��к�������Ԫ�أ�Fλ��B��ǰһ���塣��ش��������⣺
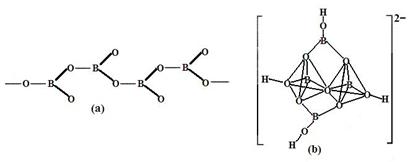
��1��Ԫ��B�����ڱ��е�λ�� ��M�Ŀռ乹���� ��
��2��A��D��E����Ԫ�����һ�ֳ��������W��û�����������Ӿ�����ͬ��ԭ���������Ŀ�Ҳ����磬W�ĵ���ʽΪ ����ҵ������ijһ����Ӧ��ͬʱ�����û������H�ĵ��ʣ�д���÷�Ӧ�Ļ�ѧ����ʽ ��
��3��E��FԪ�ص�����������Ӧ��ˮ����֮�䷴Ӧ�����ӷ���ʽ ��
��4��M��N���ܽ��H+�����н��H+������ǿ���� ���ѧʽ���������ӷ���ʽ֤�� ��
��5��E�ֱ���D��G�γ�Ħ��������ȵĻ�����X��Y������Y��ˮ��Һ�Լ��Ե�ԭ���� �������ӷ���ʽ��ʾ����������7.8 g X��ˮ��Ӧ�ų�Q kJ������Q��0����д���÷�Ӧ���Ȼ�ѧ����ʽ ��
��1����2���ڢ�A�壻 ������
��2��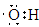 ��
��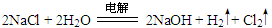
��3��Al(OH)3 + OH��==AlO2�� + 2H2O
��4��NH3��NH3 + H3O+ ="=" NH4+ + H2O
��5��S2��+ H2O HS�� + OH�� ��2Na2O2(s) + 2H2O��1��="=4NaOH(aq)" + O2(g) ��H=��20Q kJ/mol
HS�� + OH�� ��2Na2O2(s) + 2H2O��1��="=4NaOH(aq)" + O2(g) ��H=��20Q kJ/mol
�������������D�ǵؿ��к�������Ԫ����ΪO(��Ԫ��)��B������������������Ӳ�����2���������ΪHe��C��S�����ԭ������������BӦΪC(̼Ԫ��)��Fλ��B��ǰһ���壬��FΪAl(��Ԫ��)����D��Gͬ���壬��GΪS(��Ԫ��)�� E��F��G��Hͬ���ڣ�HΪCl(��Ԫ��)��A�ֱ���C��D���γɺ���10�����ӵĹ��ۻ�����M��N����AΪH(��Ԫ��)��CΪN(��Ԫ��)��MΪNH3��NΪH2O��A��Eͬ���壬��EΪNa��
��1��Ԫ��BΪ̼Ԫ�������ڱ���λ�ڵ�2���ڢ�A�壬MΪNH3���ռ乹���������Ρ�
��2��A��D��E����Ԫ�����һ�ֳ���������ΪNaOH���û������������ΪOH-�����������ͬ��ԭ���������Ŀ�Ҳ����������Ϊ�ǻ�(��OH)�������ʽΪ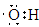 ����ҵ�����õ�ⱥ��ʳ��ˮ����NaOH����������Ӧ�Ļ�ѧ����ʽΪ
����ҵ�����õ�ⱥ��ʳ��ˮ����NaOH����������Ӧ�Ļ�ѧ����ʽΪ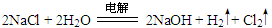 ��
��
��3��E��FԪ�ص�����������Ӧ��ˮ����ֱ�ΪNaOH��Al��OH��3�����߷�Ӧ�����ӷ���ʽΪAl(OH)3 + OH��==AlO2�� + 2H2O
��4��MΪNH3��NΪH2O������NH3�����H+����Ϊ�ɷ�����ӦNH3 + H3O+ ="=" NH4+ + H2O��
��5��E�ֱ���D��G�γ�Ħ��������ȵĻ�����X��Y�ֱ�ΪNa2O2��Na2S������Na2S��ˮ��Һ�Լ��Ե�ԭ����������ˮ�⣬���ӷ���ʽΪS2��+ H2O HS�� + OH�� ��������7.8 g Na2O2���ʵ���Ϊ0.1mol����ˮ��Ӧ�ų�Q kJ������Q��0�����ʸ÷�Ӧ���Ȼ�ѧ����ʽΪ2Na2O2(s) + 2H2O��1��="4NaOH(aq)" + O2(g) ��H=��20Q kJ/mol��
HS�� + OH�� ��������7.8 g Na2O2���ʵ���Ϊ0.1mol����ˮ��Ӧ�ų�Q kJ������Q��0�����ʸ÷�Ӧ���Ȼ�ѧ����ʽΪ2Na2O2(s) + 2H2O��1��="4NaOH(aq)" + O2(g) ��H=��20Q kJ/mol��
���㣺����Ԫ�ص��ƶϡ�����ʽ�����ӷ���ʽ���Ȼ�ѧ����ʽ��д��֪ʶ��

�±���Ԫ�����ڱ���һ���֣����������Ԫ�أ�������и�С�⡣
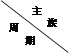 | ��A | ��A | ��A | ��A | ��A | ��A | ��A |
| 2 | | | | C | N | O | |
| 3 | Na | | Al | Si | | S | Cl |
21. ��3�����н�������ǿ��Ԫ���� ����Ԫ�����ƣ���
22. C��N��Oԭ�Ӱ뾶��С�����˳����� ��
23. ��3��������̬�⻯�����ȶ����� ���ѧʽ����
24. Si�Ǵ���������������ҪԪ��֮һ���������ﻯѧʽ�� ��
25. ���Ǵ���Ȼ��������ı������һ����;�Ƿ������ȷ�Ӧ��ұ��ijЩ���۽�����д��
�÷�Ӧ��һ����ѧ����ʽ ��
26. SԪ���γɵ��⻯������������SO2������������� ��
 ����
���� ����Ŀ֮��Ϊ________��B��C��E����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ_________(��ʵ��Ԫ�ط��ű�ʾ)��
����Ŀ֮��Ϊ________��B��C��E����Ԫ�صĵ�һ�������ɴ�С��˳��Ϊ_________(��ʵ��Ԫ�ط��ű�ʾ)�� ������Bԭ�ӹ�����ӻ�����Ϊ__________��
������Bԭ�ӹ�����ӻ�����Ϊ__________��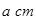 ���þ�����ܶ�Ϊ________
���þ�����ܶ�Ϊ________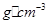 g(NA��ʾ�����ӵ�������E�����ԭ������Ϊb)��
g(NA��ʾ�����ӵ�������E�����ԭ������Ϊb)��

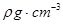 �������ӵ�����Ϊ
�������ӵ�����Ϊ �����߳�Ϊ_______cm(�ú�
�����߳�Ϊ_______cm(�ú�  ��
��