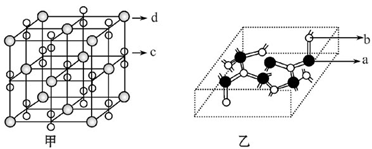
��Ŀ����
(12��)����ѧһ���ʽṹ�����ʡ�
��֪A��B��C��D��Ϊǰ������Ԫ����ԭ��������������Ԫ��A�Ļ�̬ԭ��2p�����3��δ�ɶԵ��ӣ�Ԫ��B��ԭ�����������������ڲ��������3����Ԫ��C��һ�ֳ�������Ϊ����ɫ��ĩ��D���ڲ���ȫ���������ӣ�������������Ϊl��
��1���ڵ�2�����У���һ�����ܴ���B��Ԫ����____�֡�
��2��A�������̬�⻯����ӵĿռ乹��Ϊ________��H2B���Ҵ��е��ܽ�ȴ���H2C����ԭ����_______��
��3��AB3���У�Aԭ�ӹ�����ӻ�������_______ ����AB3��Ϊ�ȵ��������Ļ�ѧʽ
Ϊ________��д��һ�ּ��ɣ���
��4��D(OH)2������ˮ�������ڰ�ˮ��д�������ڰ�ˮ�����ӷ���
ʽ_______��
( 5)D2B�ľ�����ͼ��ʾ����֪������ܶ�Ϊ 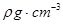 �������ӵ�����Ϊ
�������ӵ�����Ϊ �����߳�Ϊ_______cm(�ú�
�����߳�Ϊ_______cm(�ú�  ��
�� ��ʽ�ӱ�ʾ)��
��ʽ�ӱ�ʾ)��
��1��3����2�������ͣ�ˮ���Ӻ��Ҵ����Ӽ���γ��������3��sp2��CO32����
��4��Cu(OH)2+4NH3?H2O [Cu(NH3)4]2++2OH��+4H2O��( 5)
[Cu(NH3)4]2++2OH��+4H2O��( 5)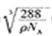 ��
��
���������������������֪��A��B��C��D��Ϊǰ������Ԫ����ԭ��������������Ԫ��A�Ļ�̬ԭ��2p�����3��δ�ɶԵ��ӣ���AΪ��Ԫ�أ�Ԫ��B��ԭ�����������������ڲ��������3������BΪ��Ԫ�أ�Ԫ��C��һ�ֳ�������Ϊ����ɫ��ĩ����CΪ��Ԫ�أ�D���ڲ���ȫ���������ӣ�������������Ϊl����DΪͭԪ�ء���1��ͬ����Ԫ���������ҵ�һ�����ܳ��������ƣ����ڢ�A��Ԫ��ԭ��p���ΪΪ�����״̬�����ȶ�����һ�����ܱ����ڵĢ�A��͢�A��Ԫ�ش��ڵ�2�����У���һ�����ܴ�������Ԫ����N��F��Ne��3�֡���2�����������̬�⻯�ﰱ���ӵĿռ乹��Ϊ�����ͣ�H2O���Ҵ��е��ܽ�ȴ���H2S����ԭ����ˮ���Ӻ��Ҵ����Ӽ���γ��������3��NO3���У�Nԭ����3�Ե��ӣ����Ӵ�1������ɣ���������ӻ�������sp2����NO3����Ϊ�ȵ��������Ļ�ѧʽΪCO32���� ��4��Cu(OH)2������ˮ�������ڰ�ˮ��������Ϸ�Ӧ�����ӷ���ʽΪCu(OH)2+4NH3?H2O [Cu(NH3)4]2++2OH��+4H2O��( 5)��Cu2O�ľ��������и������1����������Cu2����4����O2����8��1/8+1=2�����þ�������=2��160/NAg���þ������ܶ�Ϊ�� g?cm-3�����߳�a=
[Cu(NH3)4]2++2OH��+4H2O��( 5)��Cu2O�ľ��������и������1����������Cu2����4����O2����8��1/8+1=2�����þ�������=2��160/NAg���þ������ܶ�Ϊ�� g?cm-3�����߳�a=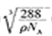 cm��
cm��
���㣺�������ʽṹ�����ʣ��漰ԭ�ӽṹ��ԭ�Ӽ�ijɼ���ʽ������Ԫ�������ɼ�����ṹ���������㡣

 ��У��������ĩ��̾�ϵ�д�
��У��������ĩ��̾�ϵ�д���10�֣�.
��. �±��г���A~R 9��Ԫ�������ڱ��е�λ��(��Ԫ�ط���)��
| ���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | | | | E | | F | | |
| 3 | A | C | D | | | | G | R |
| 4 | B | | | | | | H | |
��2�� A��B��C����Ԫ�ذ�ԭ�Ӱ뾶�ɴ�С��˳������Ϊ
��3�� FԪ���⻯��Ļ�ѧʽ��
��4�� GԪ�ظ�BԪ���γɻ�����ĵ���ʽ��
��5�� GԪ�غ�HԪ�����ߺ˵����֮����
�� ����ԭ�ӽṹ���й�֪ʶ��Ԫ�������ɡ�˼�����ش��й�114��Ԫ�صļ������⡣
ԭ�Ӻ�����_____�����Ӳ㣬����������������__________
���ڱ���λ��__________���ڣ�________��
��3�� ����_________Ԫ�أ��������ǽ�����
[���ʽṹ�����ʣ�13��]
��1����ͭͬ���ڡ���̬ԭ��������������ͬ�Ĺ���Ԫ�أ����̬ԭ�ӵĵ����Ų�ʽ ��
��2����ͼ���߱�ʾ���ֶ�����Ԫ�ص�ԭ��������������˳�����У����䳣�����ʷе�Ĺ�ϵ������A���ʾ�ĵ����� ���ѧʽ����
| ����/(pm) | B��F | B��Cl | B��Br |
| ����ֵ | 152 | 187 | 199 |
| ʵ��ֵ | 130 | 175 | 187 |
��4������Ʒ���Ӽ��������������Na+���������������ɵģ�ij�ֶ��������Ľṹ��ͼ��
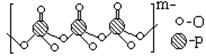
����ԭ�ӵ��ӻ�����Ϊ ��
�����ֶ�������ƵĻ�ѧʽΪ ��
��5����֪HF��F��ͨ�������ϳ�HF
 ���ж�HF
���ж�HF ��HF
��HF �����ܷ��γ��������˵�����ɡ�
�����ܷ��γ��������˵�����ɡ���
��15�֣���֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵�����������ӡ������Ϣ���±���ʾ�������ƶϻش��������⣺������ʱA��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ��
| A | A������������Ӧ��ˮ���ﻯѧʽΪH2AO3 |
| B | BԪ�صĵ�һ�����ܱ�ͬ������������Ԫ�ض��� |
| C | Cԭ����ͬ����ԭ���а뾶���ϡ��������⣩���䵥����ɫΪ��ɫ |
| D | Z�Ļ�̬ԭ�����������Ų�ʽΪ3s23p2 |
| E | E��Cλ�ڲ�ͬ���ڣ�Eԭ�Ӻ���������������C��ͬ�����������Ӿ����� |
��2��A��B��D����Ԫ�ص縺���ɴ�С����˳��Ϊ��������������������A������Ȼ��ﹹ�ɾ��������������Ϊ������������������������������
��3��A��B������⻯���н��ȶ����������������ѧʽ����B������⻯���E�ĺ�ɫ����������ڼ���ʱ�ɷ�Ӧ��д���䷴Ӧ����ʽ������������������������������������
��4)E�ĵ��ʺ���������ϡ�����пɷ�Ӧ�����˽������Ӧ��Ƴ�ԭ��أ���д��������Ӧ����ʽ��������������������������������������������
��5��úȼ�ղ�������������B����������������صĻ������⣬��ˣ�����AH4����ԭ��������Ⱦ����֪��
�� AH4(g)+2 BO2��g)�� B2(g)+AO2(g)+2H2O (g) ��H1����867kJ��mol
�� 2BO2(g) ?B2O4(g) ��H2=��56.9 kJ��mol
д��AH4��B2O4��Ӧ���Ȼ�ѧ����ʽ������������������������������������������������
�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ١����ڱ��е�λ�ã��ش��������⣺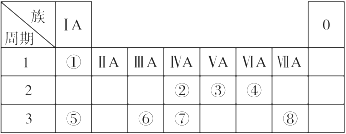
��1��������Ԫ�ص�ԭ���У�ԭ�Ӱ뾶������ ����Ԫ�ط��ţ���
��2���ؿ��к������ڵڶ�λ��Ԫ�������ڱ��е�λ���� ��
��3���١��ܡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����ۼ������ӻ����д�����ֻ�����ĵ���ʽ �� ��
��4��W�ǵ����������ͬ�����Ԫ�ء��ݴ��Ʋ�W�����ܾ��е������� ��
| A����������ϼ�Ϊ��6�� | B����̬�⻯���H2S�ȶ� |
| C������������ˮ��������Ա������� | D�������ڳ����¿����������� |

X��Һ��Y��Һ��Ӧ�����ӷ���ʽ ��
M�������ӵļ������� ��