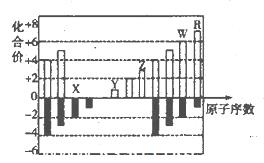
��Ŀ����
����ѧ��ѡ��3�����ʽṹ�����ʡ���15�֣�
Ԫ�����ڱ����о�Ԫ��ԭ�ӽṹ�����ʵ���Ҫ���ߡ�����X��Y��Z����Ԫ�أ���ԭ���������μ�С��XԪ��ԭ�ӵ�4p�������3��δ�ɶԵ��ӣ�YԪ��ԭ�ӵ������2p�������2��δ�ɶԵ��ӣ�X��Y���γɻ�����X2Y3��ZԪ�ؼȿ����γ���һ������Ҳ���γɸ�һ�����ӡ���ش��������⣺
��1��YԪ��ԭ�ӵļ۲���ӵĹ����ʾʽΪ______________����Ԫ�ص�������_____��
��2����X��Z�γɵĻ�����XZ3�У�X���ӻ������� ���û�����Ŀռ乹��Ϊ_____________��������Ԫ���γɵĻ���������XZ3��Ϊ�ȵ�������� ��
��3����д��X�����ֺ�����Ļ�ѧʽ �� ���������Խ�ǿ���� ��
��4��Q��Zͬ���塣Q���ʵľ�������ͼ��ʾ������þ������ܶ�Ϊag/cm3�������ӵ�����ΪNA��Qԭ�ӵ�Ħ������ΪM�����ʾQԭ�Ӱ뾶�ļ���ʽΪ ��
��15�֣�
��1��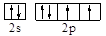 ��
�� ��2�֣� ������1�֣�
��2�֣� ������1�֣�
��2��sp3��2�֣��������Ρ���1�֣�NH3��PH3��2�֣�ÿ��1�֣�
��3��H3AsO3��H3AsO4������2�֣���1�֣�H3AsO4��1�֣�
��4�� 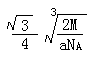 ��4�֣�ֻҪ�����ʽ�Ӽ���4�֣�����λ��Դ��������ǡ���
��4�֣�ֻҪ�����ʽ�Ӽ���4�֣�����λ��Դ��������ǡ���
�������������XԪ��ԭ�ӵ�4p�������3��δ�ɶԵ��ӣ�����X�ǵ������ڵ�������Ԫ��AsԪ�أ�YԪ��ԭ�ӵ������2p�������2��δ�ɶԵ��ӣ���2p�ϵĵ��ӿ�����2��Ҳ������4��������YԪ�ؿ�����C��O��X��Y���γɻ�����X2Y3��˵��Y�Ļ��ϼ�Ϊ-2�ۣ�����Y��OԪ�أ�ZԪ�ؼȿ����γ���һ������Ҳ���γɸ�һ�����ӣ���Z��HԪ�ء�
��1��YԪ��ԭ�������6�����ӣ����Լ۵��ӵĹ����ʾʽΪ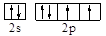 ����Ԫ����O��
����Ԫ����O��
��2����X��Z�γɵĻ�����XZ3�У���AsH3��As�ļ۲���Ӷ���=3+1/2(5-3)=4,����As��sp3�ӻ����ռ乹��Ϊ�����ͣ�������Ԫ���γɵĻ���������XZ3��Ϊ�ȵ��������ͬ����Ԫ�ص��⻯��NH3��PH3��
��3��As�����ֺ�����Ļ�ѧʽΪH3AsO3��H3AsO4����������ǿ�����ж����ݣ�ͬ��Ԫ�صĻ��ϼ�Խ�ߣ��京���������Խǿ������H3AsO4������ǿ��
��4����ͼ��֪���þ�����Qԭ�ӵĸ�����8��1/8+1=2���辧�����ⳤΪxcm,ԭ�Ӱ뾶Ϊrcm����4r=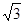 x��������֪��a=2M/NAx3���ɼ����x������r=
x��������֪��a=2M/NAx3���ɼ����x������r=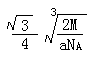
���㣺����Ԫ���ƶϣ����ʽṹ�����ʣ���������

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д���15�֣�A��B��C��D��EΪԭ���������������Ԫ�أ�����ֻ��E�����ڶ����ڣ������Ϣ���±���
| Ԫ�� | A | B | C | D | E |
| ��� ��Ϣ | �������������۴�����Ϊ2 | ��Ԫ��C���γ����Ӹ�����Ϊ2��1��1��1�Ļ����� | ����������ͨ��������ú���� | DԪ�ؿ��γ��������������һ�����γ��������Ҫ�ɷ� | �䵥������;��㷺�Ľ���������ȱ�ٸ�Ԫ����ƶѪ֢ |
��1��C��Ԫ�����ڱ��е�λ���� ��
��2��B��DԪ�ض�Ӧ����Է���������С���⻯�����ȷֽ������¶�B D�����������������������
��3������E3+���ӵķ����� ��
��4������D��������������Լ��� ������һ�֣������³�ѹ��DO2��һ����̼��Ӧ���� 1.6g D��������һ����������ų�14.86kJ��������д���˷�Ӧ���Ȼ�ѧ����ʽ ��
��5��0.1mol��L-1C2D��Һ�и�������Ũ�ȴӴ�С��˳���� ��
��6��AO2��O2������NaAO3������ȼ�ϵ�أ���ԭ����ͼ��ʾ���õ����ʹ�ù��̵缫������������Y��д���缫��ķ�Ӧʽ ��

(12��) ���Ʒ��ǿ�ѧѧϰ����Ҫ����֮һ
���������ƽ�����ȷ����
| | ��ȶ��� | ���� |
| A | Cl2+H2O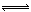 HCl+HClO HCl+HClO | I2+H2O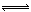 HI+HIO HI+HIO |
| B | C+2CuO ="==" 2Cu+CO2�������������ȣ� | C+SiO2 ="==" Si+ CO2�������������ȣ� |
| C | Na2O+H2O ="=" 2NaOH | CuO+H2O ="=" Cu(OH)2 |
| D | Ca(ClO)2+CO2+H2O="=" CaCO3��+2HClO | Ca(ClO)2+SO2+H2O="=" CaSO3��+2HClO |
| Ԫ�� | 8O | 16S | 34Se | 52Te |
| �����۵�(��) | ��218.4 | 113 | | 450 |
| ���ʷе�(��) | ��183 | 444.6 | 685 | 1390 |
| ��Ҫ���ϼ� | ��2 | ��2��+4��+6 | ��2��+4��+6 | |
| ԭ�Ӱ뾶 | ������ | |||
| ������H2��Ӧ��� | ��ȼʱ���� | ���Ȼ��� | �����ѻ��� | ����ֱ�ӻ��� |
�� �ڵĻ��ϼۿ��ܵķ�Χ_______ ��
�� �������ڵ��⻯��ˮ��Һ��������ǿ������˳���� (�ѧʽ)��
�� �������н�ǿ��________(������ԡ���ԭ�ԡ�)����˷��ڿ����г��ڱ����ױ��ʣ�����ܷ����Ļ�ѧ����ʽΪ___________________________________��
��24�֣��±���Ԫ�����ڱ���һ���֣���Ա��Т١�����Ԫ�أ���д���пհף�
| ���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0�� |
| 2 | | | | �� | �� | �� | �� | |
| 3 | �� | �� | �� | | | | �� | |
| 4 | �� | �� | | | | | | |
��2��������������ˮ�����У�������ǿ�Ļ�����Ļ�ѧʽ�� ��������ǿ�Ļ�����Ļ�ѧʽ�� ��
��3������������������������Ԫ���ڵ� �壻д������������������������Һ��Ӧ�����ӷ���ʽ ��
��4���Ӣݵ����Ԫ���У� ԭ�Ӱ뾶�����Ԫ�ط��ţ���
��5��Ԫ�آ�����γɵĻ��������� ���� �����ۡ������ӡ��������
��6����Ҫ�ȽϢݱȢĽ�����ǿ��������ʵ�鷽�����е��� ��
A�������ʢ����ڢ�����Һ�У�����ݲ����û������ʢޣ�˵���ݵĽ�������
B���ȽϢݺ͢�����������Ӧˮ�����ˮ���ԣ�ǰ�߱Ⱥ����ܽ�ȴ�ǰ�߽�����ǿ
C�����ݡ��ĵ��ʷֱ�Ͷ�뵽ˮ�У��۲쵽����ˮ��Ӧ�����ң�˵���ݵĽ�����ǿ
D�����ݡ��ĵ��ʷֱ���O2��ȼ�գ�ǰ�ߵõ����������ɫ�Ⱥ��ߵõ����������ɫ���ǰ�߽�����ǿ