��Ŀ����
��15�֣��������ʹ���������������õ���Ҫ�о�����
��1��������A(H3BNH3)��һ��DZ�ڵĴ�����ϣ�������Ԫ��״����(HB��NH)3ͨ�����·�Ӧ�Ƶã�3CH4��2(HB��NH)3��6H2O��3CO2��6H3BNH3����ش�
��H3BNH3���Ƿ������λ�� ����ǡ�����B��C��N��O��һ�������ɴ�С��˳��Ϊ ��CH4��H2O��CO2�����Ӱ��ռ����ɴ�С��˳������Ϊ ��
����(HB��NH)3��Ϊ�ȵ�����ķ���Ϊ �������ʽ��
���˹����Ժϳ����һϵ���⻯��������������������ƣ��ʳ�֮Ϊ���顣��ҵ�Ͽɲ���LiAlH4��BCl3��һ���������Ʊ�������B2H6���÷�Ӧ�Ļ�ѧ����ʽΪ ��
�����������У�����������״����״���Ǽ�״�ȶ��ֽṹ��ʽ��ͼ��Ϊһ��������״�ṹ�Ķ���������仯ѧʽΪ ��ͼ��Ϊ��ɰ�����������ӣ�������ԭ�Ӳ�ȡ���ӻ���ʽΪ ��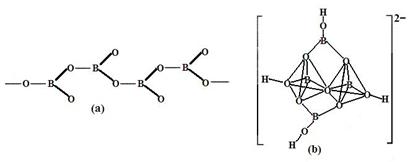
��2��һ��ͭ�Ͻ���д����
��Cu2+�ļ۲�����Ų�ʽΪ ��
��ͭ����������������仯���ﶼ���Է�����ɫ��Ӧ����ԭ���� ��
��ͭ�ĵ����а�ABCABC������ʽ�ѻ�����ͭԭ�Ӱ뾶Ϊa pm����þ�����ܶ�Ϊ g/cm3������٤������ֵΪNA��
��1�����ǣ�N>O>C>B CO2>CH4>H2O;��C6H6 ����4BCl3+3LiAlH4=2B2H6+3LiCl+3AlCl3
����4BCl3+3LiAlH4=2B2H6+3LiAlCl4����BO2--��sp3��sp2����2����3d9���ڼ���̬�ĵ��Ӵ������ϸߵĹ��ԾǨ�������ϵ͵Ĺ��ʱ����һ�������Ĺ����ʽ�ͷ���������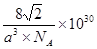 ��
��
���������������1������H3BNH3�е�B��Nԭ��֮�������λ����B��C��N��O��ͬһ���ڵ�Ԫ�ء�һ������£�Ԫ�صķǽ�����Խǿ��ԭ�Ӱ뾶ԽС��Ԫ�صĵ�һ�����ܼ���Խ��������Nԭ�ӵ�2p����ϵĵ��Ӵ��ڰ�������ȶ�״̬������������ܱ�O������˵�һ�������ɴ�С��˳��N>O>C>B��CH4����������ṹ������109��28�䣻H2O��V�ͷ��ӣ�����104.3�㣬CO2��ֱ���ͷ��ӣ�����Ϊ180�㡣����������Ӱ��ռ����ɴ�С��˳������ΪCO2>CH4>H2O���ڵȵ�������ԭ�����ȣ�����������Ҳ��ȵ����ʡ���(HB��NH)3��Ϊ�ȵ�����ķ���ΪC6H6 ���۸�������ɵúϳ�������B2H6���Ļ�ѧ����ʽΪ4BCl3+3LiAlH4=2B2H6+3LiCl+3AlCl3��Ҳ��д��4BCl3+3LiAlH4=2B2H6+3LiAlCl4���ܶ�������Ļ�ѧʽΪBO2--����ͼ����ԭ�Ӳ�ȡ���ӻ���ʽΪsp3��sp2����2����Cu��29��Ԫ�أ�Cu2+�ļ۲�����Ų�ʽΪ3d9����ͭ����������������仯���ﶼ���Է�����ɫ��Ӧ��ԭ����������ʱԭ���еĵ��������������ӻ�̬ԾǨ������̬�����Ǽ���̬�Dz��ȶ��ģ�����̬�ĵ��Ӵ������ϸߵĹ��ԾǨ�������ϵ͵Ĺ��ʱ����һ�������Ĺ����ʽ�ͷ���������Cu�������������ܶѻ�����һ�������к��е�Cuԭ�ӵĸ���Ϊ��8��1/8+6��1/2="4;" ��ͭԭ�Ӱ뾶Ϊa pm.�����ı߳�L.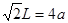 ;L=2
;L=2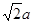 ;�þ�����ܶ�Ϊ
;�þ�����ܶ�Ϊ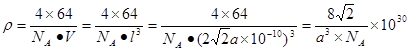 g/cm3.
g/cm3.
���㣺������λ����ԭ�ӵ��ӻ���ʽ���ȵ����塢�����ܵıȽϡ���ɫ��Ӧ��ԭ�����ܶȵļ��㡣

��10�֣��±�ΪԪ�����ڱ��е�һ���֣��û�ѧʽ��Ԫ�ط��Żش��������⣺
| | IA | ��A | ��A | ��A | VA | ��A | ��A | 0 |
| 2 | | | | �� | | �� | | |
| 3 | �� | �� | �� | | | | �� | �� |
| 4 | �� | �� | | | | | �� | |
��2���ڢ٢ڢݵ�����������ˮ�����У�������ǿ����__________���ѧʽ����
��3��Ԫ�آߵij������⻯��Ļ�ѧʽΪ__ __�����⻯�ﳣ���º�Ԫ�آڵĵ��ʷ�Ӧ�����ӷ���ʽ�ǣ�_______________________�����⻯����Ԫ�آ�ĵ��ʷ�Ӧ�����ӷ���ʽ��___________________________��
��4���ٺ͢������������Ӧ��ˮ���ﻯѧʽ�ֱ�Ϊ___________��_______��
��5���ٺ͢�����������Ӧ��ˮ�������Ӧ�Ļ�ѧ����ʽΪ__________________
��6�������ֱ���H2�γɵ��⻯����ȶ��ԣ�__________�����û�ѧʽ��ʾ������������Ӧ��ˮ��Һ�����ԣ�_______________�����û�ѧʽ��
��15�֣���֪A��B��C��D��E�������ڱ���ǰ�����ڵ�Ԫ�أ����ǵĺ˵�����������ӡ������Ϣ���±���ʾ�������ƶϻش��������⣺������ʱA��B��C��D��E������Ӧ��Ԫ�ط��ű�ʾ��
| A | A������������Ӧ��ˮ���ﻯѧʽΪH2AO3 |
| B | BԪ�صĵ�һ�����ܱ�ͬ������������Ԫ�ض��� |
| C | Cԭ����ͬ����ԭ���а뾶���ϡ��������⣩���䵥����ɫΪ��ɫ |
| D | Z�Ļ�̬ԭ�����������Ų�ʽΪ3s23p2 |
| E | E��Cλ�ڲ�ͬ���ڣ�Eԭ�Ӻ���������������C��ͬ�����������Ӿ����� |
��2��A��B��D����Ԫ�ص縺���ɴ�С����˳��Ϊ��������������������A������Ȼ��ﹹ�ɾ��������������Ϊ������������������������������
��3��A��B������⻯���н��ȶ����������������ѧʽ����B������⻯���E�ĺ�ɫ����������ڼ���ʱ�ɷ�Ӧ��д���䷴Ӧ����ʽ������������������������������������
��4)E�ĵ��ʺ���������ϡ�����пɷ�Ӧ�����˽������Ӧ��Ƴ�ԭ��أ���д��������Ӧ����ʽ��������������������������������������������
��5��úȼ�ղ�������������B����������������صĻ������⣬��ˣ�����AH4����ԭ��������Ⱦ����֪��
�� AH4(g)+2 BO2��g)�� B2(g)+AO2(g)+2H2O (g) ��H1����867kJ��mol
�� 2BO2(g) ?B2O4(g) ��H2=��56.9 kJ��mol
д��AH4��B2O4��Ӧ���Ȼ�ѧ����ʽ������������������������������������������������
��24�֣��±���Ԫ�����ڱ���һ���֣���Ա��Т١�����Ԫ�أ���д���пհף�
| ���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0�� |
| 2 | | | | �� | �� | �� | �� | |
| 3 | �� | �� | �� | | | | �� | |
| 4 | �� | �� | | | | | | |
��2��������������ˮ�����У�������ǿ�Ļ�����Ļ�ѧʽ�� ��������ǿ�Ļ�����Ļ�ѧʽ�� ��
��3������������������������Ԫ���ڵ� �壻д������������������������Һ��Ӧ�����ӷ���ʽ ��
��4���Ӣݵ����Ԫ���У� ԭ�Ӱ뾶�����Ԫ�ط��ţ���
��5��Ԫ�آ�����γɵĻ��������� ���� �����ۡ������ӡ��������
��6����Ҫ�ȽϢݱȢĽ�����ǿ��������ʵ�鷽�����е��� ��
A�������ʢ����ڢ�����Һ�У�����ݲ����û������ʢޣ�˵���ݵĽ�������
B���ȽϢݺ͢�����������Ӧˮ�����ˮ���ԣ�ǰ�߱Ⱥ����ܽ�ȴ�ǰ�߽�����ǿ
C�����ݡ��ĵ��ʷֱ�Ͷ�뵽ˮ�У��۲쵽����ˮ��Ӧ�����ң�˵���ݵĽ�����ǿ
D�����ݡ��ĵ��ʷֱ���O2��ȼ�գ�ǰ�ߵõ����������ɫ�Ⱥ��ߵõ����������ɫ���ǰ�߽�����ǿ
�±�ΪԪ�����ڱ���һ���֣������Ԫ�آ١����ڱ��е�λ�ã��ش��������⣺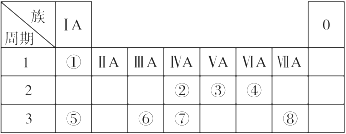
��1��������Ԫ�ص�ԭ���У�ԭ�Ӱ뾶������ ����Ԫ�ط��ţ���
��2���ؿ��к������ڵڶ�λ��Ԫ�������ڱ��е�λ���� ��
��3���١��ܡ����е�ijЩԪ�ؿ��γɼȺ����Ӽ��ֺ����ۼ������ӻ����д�����ֻ�����ĵ���ʽ �� ��
��4��W�ǵ����������ͬ�����Ԫ�ء��ݴ��Ʋ�W�����ܾ��е������� ��
| A����������ϼ�Ϊ��6�� | B����̬�⻯���H2S�ȶ� |
| C������������ˮ��������Ա������� | D�������ڳ����¿����������� |

X��Һ��Y��Һ��Ӧ�����ӷ���ʽ ��
M�������ӵļ������� ��
�±���Ԫ�����ڱ���һ����, ��Ա��еĢ١�����Ԫ��,��д���пհ�:
| ���� ���� | ��A | ��A | ��A | ��A | ��A | ��A | ��A | 0 |
| 2 | | | | �� | �� | �� | | |
| 3 | �� | | �� | | | �� | �� | �� |
| 4 | �� | | | | | | | |
��1������ЩԪ����,��ѧ��������õ���: (����廯ѧ����,��ͬ)��
��2��������������ˮ�����У�������ǿ�Ļ�����Ļ�ѧʽ�� ��������ǿ�Ļ�����ĵ���ʽ�ǣ� ��
��3������������������������Ԫ���� ��д���������������������Ʒ�Ӧ�����ӷ���ʽ ��
��4�����⻯����۵ĵ�����һ�������·�Ӧ�Ļ�ѧ����ʽΪ�� ��
��5�� �ڿ����γɶ������������һ���Ǻ���ɫ���壬���÷���ʽ˵�������岻�˲�����ˮ���ռ���ԭ�� ��
��6�� �ýṹʽ��ʾԪ�آ�����γɵĻ����� ���û������ڹ���ʱ�׳� ������ ���壬ָ������һ����; ��
���ֶ�����Ԫ��W��X��Y��Z��ԭ�����������������ϱ�����Ϣ�ش��������⡣
| | W | X | Y | Z |
| �ṹ������ | ����������Ӧ��ˮ����������̬�⻯�ﷴӦ�õ����ӻ����� | ��ɫ��Ӧ�ʻ�ɫ | ��ͬ��������Ԫ���γɵļ������У����Ӱ뾶��С | ��������������֮��Ϊ�� |
��1��Z��Ԫ�����ڱ���λ�� �塣
��2������Ԫ�ص�����������Ӧ��ˮ�����У���һ��������һ�������¾����������������ʷ�����ѧ��Ӧ����Ԫ���� (��Ԫ�ط���)��
��3�������п���Ϊ�Ƚ�X��Y������ǿ���������� ������ţ���
A����Ȼ���еĺ��� B���������ᷴӦʱʧȥ�ĵ�����
C��������ˮ��Ӧ�����׳̶� D������������Ӧˮ����ļ���
�ڴ�ԭ�ӽṹ�ĽǶȽ���X�Ľ�����ǿ��Y��ԭ�� ��ԭ�Ӱ뾶X>Y������ԭ�Ӻ˶��������ӵ�������X<Y��ʧ��������X>Y��
��4��W��һ���⻯��HW3�������л��ϳɣ���������������ơ������Ũ�Ⱦ���ȵ�HW3��X������������Ӧ��ˮ�������Һ��ϣ���Ӧ�Ļ�ѧ����ʽ�� ��
��5��Y���ʺ�Mg��ɵĻ������һ�����ԭ�ϣ�ij��ȤС�����������ʾ��ʵ�鷽�����ⶨ�������Y��������������ȷ���������Y������������������ (�����)��
A��m��n B��m��y C��n��y
