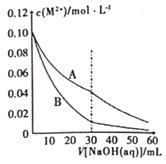
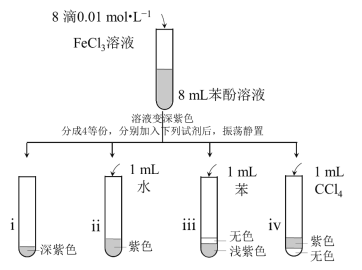
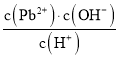��Ŀ����
����Ŀ����ҵ��ˮ�к��У��̡������ӵ��ؽ���Ԫ�ء�������ҵ��ˮ�к��е�Cr2O72-��CrO42-�����õķ���Ϊ��ԭ���������÷��Ĺ�������Ϊ:
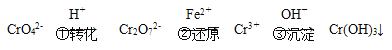
���еڢٲ�����ƽ��2CrO42- (��ɫ)��2H��![]() Cr2O72- (��ɫ)��H2O��
Cr2O72- (��ɫ)��H2O��
(1)д���ڢٲ���Ӧ��ƽ�ⳣ������ʽ____________________________________��
(2)���ڵڢٲ���Ӧ������˵����ȷ����________��
A��ͨ���ⶨ��Һ��pH�����жϷ�Ӧ�Ƿ��Ѵﵽƽ��״̬
B���÷�ӦΪ������ԭ��Ӧ
C��ǿ���Ի�������Һ����ɫΪ��ɫ
(3)�ڢڲ��У���ԭ0.1 mol Cr2O72-����Ҫ________ mol��FeSO4��7H2O��
(4)�ڢ۲�������Cr(OH)3�⣬���������ɵij���Ϊ________��
(5)����Һ�д������³����ܽ�ƽ�⣺Cr(OH)3(s) ![]() Cr3��(aq)��3OH��(aq)�������£�Cr(OH)3���ܶȻ�Ksp��10��32����c(Cr3��)����10��5 mol/L����Ϊc(Cr3��)�Ѿ���ȫ�������ֽ��ڢ۲���Һ��pH����4����ͨ������˵��Cr3���Ƿ������ȫ(��д���������)��______________________________________��
Cr3��(aq)��3OH��(aq)�������£�Cr(OH)3���ܶȻ�Ksp��10��32����c(Cr3��)����10��5 mol/L����Ϊc(Cr3��)�Ѿ���ȫ�������ֽ��ڢ۲���Һ��pH����4����ͨ������˵��Cr3���Ƿ������ȫ(��д���������)��______________________________________��
���𰸡�(1)K��![]()
(2)AC
(3)0.6
(4)Fe(OH)3
(5)��pH����4ʱ��c(OH��)��10��10mol/L��c(Cr3��)��10��32/c3(OH��) ��10��2mol/L��10��5mol/L�����Cr3��û�г�����ȫ
��������
(1)��H2O�Ǵ�Һ̬���ʣ���Ũ����Ϊ�������ʵ�������Ӧ��ƽ�ⳣ������ʽΪK��c(Cr2O72-)/[c2(CrO42-)��c2(H��)]��
(2)���÷�Ӧ����֪����Ӧ�������ʱ����ҺpH����pH����ʱ˵����Ӧ�ﵽƽ�⣬A����ȷ��CrO42-��Cr2O72-��Cr��Ϊ��6�ۣ��÷�Ӧ����������ԭ��Ӧ��B����������Ի����У���Һ��c(Cr2O72-)�ϴ���Һ�ʳ�ɫ��C����ȷ��
(3)�ڵ�������Ӧ��Cr2O72-����ԭΪCr3����0.1 mol Cr2O72-����ԭʱת�Ƶ��ӵ����ʵ���Ϊ0.1 mol��2��(6��3)��0.6 mol������ԭ��Fe2��������ΪFe3��������Ҫ����0.6 mol FeSO4��7H2O��
(4)�ڵ�������Ӧ��Fe2��������ΪFe3�����ʵ���������Fe(OH)3���ɡ�

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ����ѧ����ᡢ����������ء��������������ʵ�Ľ��Ͳ���ȷ����( )
ѡ�� | �������ʵ | ���ͻ��Ӧ�����ӷ���ʽ |
A | ���ȵĴ�����Һϴȥ���� | CO32��+H2O ��Һ�ʼ��ԣ��¶���������ǿ |
B | ������Ʒ�ڿ����з���pH��С | SO2+H2O=H2SO3 |
C | ����ĭ�������� | Al3+ + 3HCO3-= Al(OH)3��+3CO2�� |
D | �ü��ȷ���ȥNaCl�����л��е�NH4Cl���� | NH4Cl���������ȫ�ֽ��Ϊ�������ȥ |
A. A B. B C. C D. D
����Ŀ���о�ͭ������Ũ����ķ�Ӧ��ʵ�����£�
�� | �� |
| |
ͭ˿�������������� ��˿����Ѹ�ٱ�ڣ�֮������������ | ͭ˿����˿���ܽ⣬�����������壬 Ʒ����Һ��ɫ |
����˵����ȷ����
A. �����²�������������ʢ��Ũ���ᣬ����ͭ������ʢ��Ũ����
B. ����ͭ˿����˿����ʣ��ʱ��������������ʵ������
C. ���ݢڣ����ƶϳ�ͭ������Ũ���ᷴӦ������SO2
D. �٢�������IJ�����������¶ȸı��˻�ѧ��Ӧ����