��Ŀ����
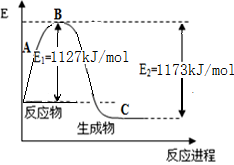
(10��) ������һ����Ҫ�Ļ���ԭ�ϣ���ҵ����N2��H2�ϳ�NH3������֪N2(g)��H2(g)��Ӧ����1 mol NH3(g)�����������仯ʾ��ͼ������ͼ���ش��������⣺
��1���÷�ӦΪ (����ȡ����ȡ�)��Ӧ��
��2���ϳɰ����Ȼ�ѧ����ʽΪ ��
��3��������֪�����������ϱ�����������������N-H������Ϊ kJ/mol��
��4����ҵ�ϣ�����Ϊԭ����������ĵ�һ��Ϊ������������д���÷�Ӧ�Ļ�ѧ����ʽ ��

��1���÷�ӦΪ (����ȡ����ȡ�)��Ӧ��
��2���ϳɰ����Ȼ�ѧ����ʽΪ ��
��3��������֪�����������ϱ�����������������N-H������Ϊ kJ/mol��
��4����ҵ�ϣ�����Ϊԭ����������ĵ�һ��Ϊ������������д���÷�Ӧ�Ļ�ѧ����ʽ ��
��1������ ��2��N2(g)+3H2(g) 2 NH3(g) ��H����92 kJ/mol
2 NH3(g) ��H����92 kJ/mol
��3��391 ��4�� 4NH3 + 5O2 4NO + 6H2O ��
4NO + 6H2O ��
 2 NH3(g) ��H����92 kJ/mol
2 NH3(g) ��H����92 kJ/mol��3��391 ��4�� 4NH3 + 5O2
 4NO + 6H2O ��
4NO + 6H2O �������������1�����ڷ�Ӧ����������������������������ʵ�����������ͷų�������˸÷�Ӧ�Ƿ��ȷ�Ӧ����2������ͼʾ��֪���ϳɰ����Ȼ�ѧ����ʽΪN2(g)+3H2(g)
 2 NH3(g) ��H����92 kJ/mol����3����Ӧ�Ⱦ��Ƕ��ѻ�ѧ�����յ��������γɻ�ѧ���ͷŵ������IJ436 kJ/mol��3mol+946 kJ/mol��1mol-6X=��92 kJ/mol,���X=391 kJ/mol����4����ҵ�ϣ�����Ϊԭ����������ĵ�һ��Ϊ�����������÷�Ӧ�Ļ�ѧ����ʽ��4NH3 + 5O2
2 NH3(g) ��H����92 kJ/mol����3����Ӧ�Ⱦ��Ƕ��ѻ�ѧ�����յ��������γɻ�ѧ���ͷŵ������IJ436 kJ/mol��3mol+946 kJ/mol��1mol-6X=��92 kJ/mol,���X=391 kJ/mol����4����ҵ�ϣ�����Ϊԭ����������ĵ�һ��Ϊ�����������÷�Ӧ�Ļ�ѧ����ʽ��4NH3 + 5O2  4NO + 6H2O��
4NO + 6H2O��
��ϰ��ϵ�д�
�����Ŀ
 2NH3(g) ��H=��92��4kJ/mol
2NH3(g) ��H=��92��4kJ/mol O2(g); ��H=" -485" kJ/mol
O2(g); ��H=" -485" kJ/mol

 2HCl��g��+O2��g����H>0��
2HCl��g��+O2��g����H>0�� O2��g��= H2O��1��, ��H2 = -285��84kJ��mol-l
O2��g��= H2O��1��, ��H2 = -285��84kJ��mol-l CH3OH(g)
CH3OH(g)