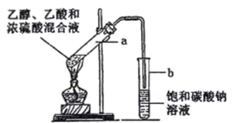
��Ŀ����
����Ŀ���̲��ں��Ĵ�������ȼ�������俪�������ǵ�ǰ�����ԴΣ������Ҫ���⡣CH4(g)+2H2O(g)![]() CO2(g)+4H2(g)��H3=+akJ��mol-1
CO2(g)+4H2(g)��H3=+akJ��mol-1
��ѧ�� | C��H | H��H | H��O |
����kJ/mol | b | c | d |
(1)д������Ľṹʽ___________
(2)C=O�ļ���Ϊ_______________kJ/mol(�ú�a��b��c��d��ʽ�ӱ�ʾ)
(3)���������£��������Ϊ0.5L���ܱ�������ͨ��һ���������ˮ����������������Ӧ����ü������ʵ�����ʱ��仯���±���ʾ��0��10min����H2O��Ũ�ȱ�ʾ�÷�Ӧ��ƽ������Ϊ��(H2O)=_____________
ʱ��/min | 0 | 10 | 20 | 40 | 50 | 60 |
n(CH4)/mol | 0.50 | 0.35 | 0.25 | 0.10 | 0.10 | 0.10 |
(4)���º�������£�����������˵���˷�Ӧ�ﵽƽ��״̬����_______________��
a����������ƽ����Է����������ֲ���b��CO2��H2�����������Ϊ1�s4
c�����������ܶȱ��ֲ���d��1molCO2���ɵ�ͬʱ��4molH-H������
(5)д������ȼ�ϵ�أ���KOH��ҺΪ����ʱ�������ĵ缫��Ӧʽ______
���𰸡�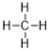 2b+2d-2c-
2b+2d-2c-![]() 0.06molL1min1 ad 10OH��+CH4��8e��=
0.06molL1min1 ad 10OH��+CH4��8e��=![]() +7H2O
+7H2O
��������
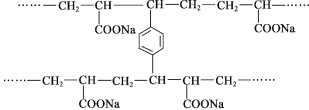
(1)����ķ���ʽΪCH4������Ϊ��������ṹ���ṹʽΪ�� ��
��
�ʴ�Ϊ�� ��
��
(2)������H=��Ӧ��ļ����ܺ�-������ļ����ܺͼ��㣬��H3=4b+4d-2E(C=O)-4c=a����ã�E(C=O)= 2b+2d-2c-![]() ��
��
�ʴ�Ϊ��2b+2d-2c-![]() ��
��
(3) 0��10min�ڼ���ķ�Ӧ���ʵ����仯��Ϊ��0.50 mol -0.35 mol =0.15mol��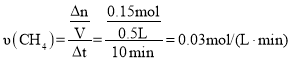 ����������֮�ȵ��ڻ�ѧ������֮�ȣ��ɵ���(H2O)=
����������֮�ȵ��ڻ�ѧ������֮�ȣ��ɵ���(H2O)= ![]() ��
��
�ʴ�Ϊ��0.06molL1min1��
(4) a����Ӧǰ����������������䣬��������ʵ������ӣ���Ӧ�����л�������ƽ����Է��������DZ��������䱣�ֲ���ʱ��Ӧ�ﵽƽ��״̬����a��˵����Ӧ�ﵽƽ��״̬��
b��CO2��H2��Ϊ�����Ӧ���������ߵ����ʵ���֮��ʼ�յ��ڻ�ѧ������֮�ȣ����������Ϊ1�s4���䣬��b����˵���÷�Ӧ�ﵽƽ��״̬��
c����Ӧ�����������������䣬����������䣬��������ܶ�ʼ�ձ��ֺ㶨����˻��������ܶȱ��ֲ��䲻��˵����Ӧ�ﵽƽ��״̬��
d��1molCO2����������Ӧ��ͬʱ��4molH-H������������Ӧ�������ʱȵ��ڻ�ѧ������֮�ȣ���˵����Ӧ�ﵽƽ��״̬��
�ʴ�Ϊ��ad��
(5)����ȼ�ϵ�ظ����Ǽ��鷴Ӧת��ɶ�����̼���ڼ�����������ת���̼������ӣ�������ӦʽΪ��10OH��+CH4��8e��=![]() +7H2O��
+7H2O��
�ʴ�Ϊ��10OH��+CH4��8e��=![]() +7H2O��
+7H2O��