��Ŀ����
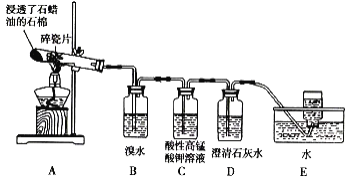
����Ŀ����ͼΪʵ������ȡ����������װ�á���ش�
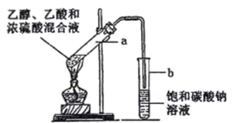
(1) д�����ᡢ�Ҵ�������Ӧ����ʽ__________
(2) Ũ�����������__________
(3) �����йظ�ʵ���˵���У���ȷ����__________
A.�� a �Թ��м����ʯ���������Ƿ�ֹ����ʱҺ�屩��
B.����̼������Һ���Գ�ȥ�����л��е�����
C.����������һ����ɫ�����ܶȱ�ˮ�����״Һ��
D.��ԭ��Ϊ CH3COOH �� CH3CH218OH�������������в���18O
���𰸡�CH3COOH+C2H5OH![]() CH3COOC2H5+H2O ��������ˮ�� AB
CH3COOC2H5+H2O ��������ˮ�� AB
��������
(1)�Ҵ���������Ũ��������������ˮ���������·���������Ӧ��������������ˮ����Ӧ�Ļ�ѧ����ʽΪ��CH3COOH+C2H5OH![]() CH3COOC2H5+H2O��
CH3COOC2H5+H2O��
(2) �������Ҵ���Ũ�������������÷�ӦΪ���淴Ӧ��Ũ������ˮ����ƽ���������������������ƶ�����Ũ���������Ϊ��������ˮ����
(3) A.���ᡢ�Ҵ��е�ͣ�Һ�����ʱҪ�ӷ�ʯ(�����Ƭ)���ɷ�ֹ��Һ���У�A��ȷ��
B.����������Ա�̼��ǿ�����������ܺ�̼���Ʒ�Ӧ�����Գ�ȥ���ᣬB��ȷ��
C.���������ܶȱ�ˮС��C����
D.������Ӧ�Ļ���Ϊ�����ǻ��������⣬������ԭ��ΪCH3COOH��CH3CH218OH�������������к�18O��D����
��ѡAB��

 ��У����ϵ�д�
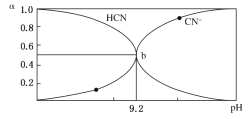
��У����ϵ�д�����Ŀ���̲��ں��Ĵ�������ȼ�������俪�������ǵ�ǰ�����ԴΣ������Ҫ���⡣CH4(g)+2H2O(g)![]() CO2(g)+4H2(g)��H3=+akJ��mol-1
CO2(g)+4H2(g)��H3=+akJ��mol-1
��ѧ�� | C��H | H��H | H��O |
����kJ/mol | b | c | d |
(1)д������Ľṹʽ___________
(2)C=O�ļ���Ϊ_______________kJ/mol(�ú�a��b��c��d��ʽ�ӱ�ʾ)
(3)���������£��������Ϊ0.5L���ܱ�������ͨ��һ���������ˮ����������������Ӧ����ü������ʵ�����ʱ��仯���±���ʾ��0��10min����H2O��Ũ�ȱ�ʾ�÷�Ӧ��ƽ������Ϊ��(H2O)=_____________
ʱ��/min | 0 | 10 | 20 | 40 | 50 | 60 |
n(CH4)/mol | 0.50 | 0.35 | 0.25 | 0.10 | 0.10 | 0.10 |
(4)���º�������£�����������˵���˷�Ӧ�ﵽƽ��״̬����_______________��
a����������ƽ����Է����������ֲ���b��CO2��H2�����������Ϊ1�s4
c�����������ܶȱ��ֲ���d��1molCO2���ɵ�ͬʱ��4molH-H������
(5)д������ȼ�ϵ�أ���KOH��ҺΪ����ʱ�������ĵ缫��Ӧʽ______
����Ŀ���±���A��B��C��D��E�����л�����й���Ϣ��
A | B | C | D | E |
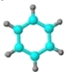
����ʹ������Ȼ�̼��Һ��ɫ�� �ڱ���ģ��Ϊ�� ������ˮ��һ�������·�Ӧ����C | ����C��H����Ԫ����ɣ� �����ģ��Ϊ�� | ����ʽΪC2H6O������E���� | ����Է���������C��2�� ������C�������ɣ� | ����C��H��O����Ԫ����ɣ� �����ģ��Ϊ�� |
���ݱ�����Ϣ�ش��������⣺
(1)A��E�У�����������__________(����ĸ)��д��A����ˮ��Ӧ�Ļ�ѧ����ʽ__________��
(2)B���������__________(�����)��
����ɫ��ζҺ�� ���ж� �۲�����ˮ ���ܶȱ�ˮС �ݿ�ʹ���Ը��������Һ����ˮ����ɫ
(3)д��C��D �ṹ��ʽ��__________��__________��
(4)E �к��������ŵ�����__________��