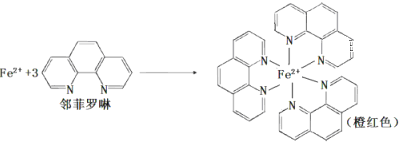
��Ŀ����
����Ŀ���±�ΪԪ�����ڱ���һ���֣������Ԫ�آ�~���ڱ��е�λ��,�ش�����:
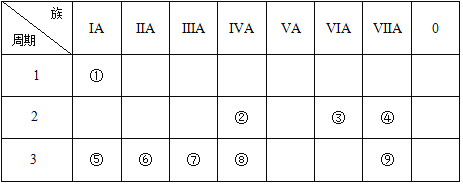
(1)�������ڰ뵼����ϵ�Ԫ�������ڱ��е�λ��__________��
(2)�ۡ��ܡ����ԭ�Ӱ뾶��С��__________(��Ԫ�ط��Żش�)��
(3)�ݡ��ޡ��ߵ�����������Ӧ��ˮ���������ǿ����________(�û�ѧʽ�ش�)��
(4)�ڡ��ۡ��ܵ���̬�⻯��ȶ�����ǿ����__________(�ýṹʽ�ش�)��
(5)�ں͢۰�ԭ����1:2�γɵĻ�����ĵ���ʽΪ____���þ��������Ĺ����п˷�������������Ϊ______��
(6)�ۺ͢��γɵĻ���������__________(�������ӻ��������������ۻ�������)���þ�������__________����(������������������������ԭ����)��
(7)Ԫ�آݡ��ߵ�����������ˮ���ﻥ�෴Ӧ�����ӷ���ʽΪ��_____________��
���𰸡���3���ڵ�IVA�� F NaOH H-F ![]() ���Ӽ������� ���ۻ����� ԭ�� Al(OH)3+OH-=AlO2- +2H2O
���Ӽ������� ���ۻ����� ԭ�� Al(OH)3+OH-=AlO2- +2H2O
��������
��Ԫ�������ڱ���λ�ÿ�֪����ΪH����ΪC����ΪO����ΪF����ΪNa����ΪMg����ΪAl����ΪSi����ΪCl�����Ԫ�������ɷ������
(1)Si�dz����İ뵼����ϣ���Ԫ�������ڱ��е�λ���ǵ�3���ڵ�IVA�壬�ʴ�Ϊ����3���ڵ�IVA�壻
(2)һ����ԣ����Ӳ���Խ�࣬�뾶Խ���Ӳ�����ͬ���˵����Խ�뾶ԽС���ʢۡ��ܡ����ԭ�Ӱ뾶��С�Ǣܣ���ΪF���ʴ�Ϊ��F��
(3)Ԫ�صĽ�����Խǿ������������Ӧ��ˮ�������Խǿ���ʢݡ��ޡ��ߵ�����������Ӧ��ˮ���������ǿ���Ǣݣ���ΪNaOH���ʴ�Ϊ��NaOH��
(4)�ǽ�����Խǿ����̬�⻯��Խ�ȶ����ʢڡ��ۡ��ܵ���̬�⻯��ȶ�����ǿ���Ǣܣ���ΪH-F���ʴ�Ϊ��H-F��
(5)�ں͢۰�ԭ����1��2�γɵĻ�����ΪCO2������ʽΪ![]() ��CO2Ϊ���Ӿ��壬�����Ĺ�������Ҫ�˷����Ӽ����������ʴ�Ϊ��
��CO2Ϊ���Ӿ��壬�����Ĺ�������Ҫ�˷����Ӽ����������ʴ�Ϊ��![]() �����Ӽ���������
�����Ӽ���������
(6)�ۺ͢��γɵĻ�����ΪSiO2���û�����Ϊ���ۻ����SiO2����Ĺ�����Ϊԭ�ӣ�����ԭ�Ӽ��ͨ�����ۼ�����γɿռ���״�ṹ�ľ��壬����ԭ�Ӿ��壬�ʴ�Ϊ�����ۻ����ԭ�ӣ�
(7)Ԫ�آݡ��ߵ�����������ˮ����ֱ�ΪNaOH��Al(OH)3�����߷�Ӧ����ƫ�����ƺ�ˮ����Ӧ�����ӷ���ʽΪAl(OH)3+OH-=AlO2- +2H2O���ʴ�Ϊ��Al(OH)3+OH-=AlO2- +2H2O��

����Ŀ�����ݱ����ṩ�IJ��ֶ�����Ԫ��ԭ�Ӱ뾶����Ҫ���ϼ۵���Ϣ���ش���������
Ԫ�ش��� | A | B | C | D | E | F | G |
ԭ�Ӱ뾶/nm | 0.186 | 0.160 | 0.143 | 0.112 | 0.104 | 0.099 | 0.066 |
��Ҫ���ϼ� | +1 | ��2 | ��3 | ��2 | ��6����2 | +7��-1 | ��2 |
��1��A��Ԫ�����ڱ���λ����____��
��2��B�����ӽṹʾ��ͼ��_____��
��3��C������A������������Ӧ��ˮ���ﷴӦ�����ӷ���ʽ��____��
��4��Ԫ������������Ӧ��ˮ����������ǿ���ǣ�____ (д��ѧʽ)��
��5��AԪ����FԪ���γɵĻ�����ĵ���ʽ _____��