��Ŀ����
����Ŀ����������������������������ͻ���ϢϢ��ء�
(1)NO���ٳ����㱻�ƻ����䷴Ӧ������ͼ��ʾ��
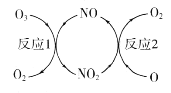
��NO��������________________________��
����֪��O3(g)+O(g) ��2O2(g) ��H=-143kJ��mol-1
��Ӧl��O3(g)+NO(g) ��NO2(g)+O2(g)��H1=-200.2kJ��mol-1
��Ӧ2���Ȼ�ѧ����ʽΪ____________________________��
(2)���쵰��(Mb)�Ǽ����ڴ������ĵ����ʣ����ɼ��쵰�ĸʰ���(H2NCH2COOH)��һ���������ʣ�����Һ��������������ʽ���ڣ���ת����ϵ���� �������ӵ����ʵ�������(��)��[
�������ӵ����ʵ�������(��)��[![]() ]�Ĺ�ϵ��ͼ��ʾ��
]�Ĺ�ϵ��ͼ��ʾ��
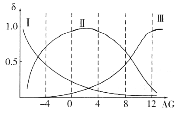
����Һ������ʱ����������Ũ���ɴ�С��˳��Ϊ______________________��
����AG=12����Һ�м������NaOH��Һʱ����Ҫ��Ӧ�����ӷ���ʽΪ______________��
(3)���쵰��(Mb)����O2�������MbO2��![]() ��37��ʱ��ü��쵰�Ľ�϶�(��)��p(O2)�Ĺ�ϵ���±���
��37��ʱ��ü��쵰�Ľ�϶�(��)��p(O2)�Ĺ�ϵ���±���
[��϶�(��)ָ����O2��ϵļ��쵰��ռ�ܼ��쵰�����ʵ�������]
p(O2)��kPa | 0.50 | 1.00 | 2.00 | 3.00 | 4.00 | 5.00 | 6.00 |
��(MbO2)% | 50.0 | 66.7 | 80.0 | 85.7 | 88.9 | 90.9 | 92.3 |
�ټ���37��ʱ��������Ӧ��ƽ�ⳣ��K=__________kPa-1(�������Һ�е����ʷֱ��÷�ѹ�����ʵ���Ũ�ȱ�ʾ)��
��37��ʱ����������������ѹΪ21.0 kPa��������������ʱ�������ֵΪ________��(����1λС��)��
���¶Ȳ���ʱ���ο���ɽ��ʱ����MbO2��Ũ�ȱ���ɽ��________(����������������)��
���о����֣�v��=k����c(Mb)��p(O
���𰸡����� NO2��g��+O��g��=NO(g)+O2(g) ��H=+57.2KJ/mol c(H3N+CH2COO��)>c��H2NCH2COO����>c(H3N+CH2COOH) H3N+CH2COOH+2OH��= H2NCH2COO��+2H2O 2 97.7 �� 120s-1 KPa-1
��������
(1)��NOֻ�Dz��뻯ѧ��Ӧ�м����̵ģ����䱾���������ͻ�ѧ�����ڷ�Ӧǰ��������ֲ��䣻
�����ø�˹���ɽ��⣻
(2)���ź����������Լ�����������ǿ������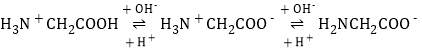 ��֪H2NCH2COO������Խǿ����Խ��H3N+CH2COO���ڽӽ�����ʱ�������H3N+CH2COOH����Խǿ����Խ���ɴ�ȷ������ΪH2NCH2COO��,����ΪH3N+CH2COO��,����ΪH3N+CH2COOH���Դ˷�����
��֪H2NCH2COO������Խǿ����Խ��H3N+CH2COO���ڽӽ�����ʱ�������H3N+CH2COOH����Խǿ����Խ���ɴ�ȷ������ΪH2NCH2COO��,����ΪH3N+CH2COO��,����ΪH3N+CH2COOH���Դ˷�����
(3)��K=![]() kPa-1�����ñ��������ݽ��м��㣻
kPa-1�����ñ��������ݽ��м��㣻
�����â��м����K���㼴�ɣ�
�۸��ݱ����ṩ���ݿ�֪p(O2)ԽС����϶�Խ�ͣ�c��MbO2��ԽС��
�����â��м����K������ƽ��ʱv��= v�����м��㣻
(1)��NOֻ�Dz��뻯ѧ��Ӧ�м����̵ģ����䱾���������ͻ�ѧ�����ڷ�Ӧǰ��������ֲ��䣬���NO�������Ǵ�����
�𰸣�����
�����ø�˹���ɣ�������֪��O3(g)+O(g) =2O2(g) ��H=-143kJ��mol-1
O3(g)+NO(g) =NO2(g)+O2(g)��H1=-200.2kJ��mol-1
ǰ��ȥ���߿ɵã�NO2��g��+O��g��=NO(g)+O2(g) ��H=+57.2kJ/mol
�𰸣�NO2��g��+O��g��=NO(g)+O2(g) ��H=+57.2kJ/mol
(2)�����ź����������Լ�����������ǿ��������ɿ�֪����ԽǿH2NCH2COO������Խ����AG=0��4��ΧʱH3N+CH2COO�������������ԽǿH3N+CH2COOH����Խ���ɴ�ȷ������ΪH2NCH2COO��,����ΪH3N+CH2COO��,����ΪH3N+CH2COOH����Һ������Ҳ����AG=0����������Ũ���ɴ�С��˳��ɸ���ͼ��õ�Ϊc(H3N+CH2COO��)>c��H2NCH2COO����>c(H3N+CH2COOH)��
�𰸣�c(H3N+CH2COO��)>c��H2NCH2COO����>c(H3N+CH2COOH)
�ڸ���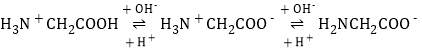 ��֪�������NaOH��Һʱ��Һ����Ҫ����H2NCH2COO-��AG=12ʱ��Һ����Ҫ����H3N+CH2COOH���ݴ�д�����ӷ���ʽΪ: H3N+CH2COOH+2OH��= H2NCH2COO��+2H2O��
��֪�������NaOH��Һʱ��Һ����Ҫ����H2NCH2COO-��AG=12ʱ��Һ����Ҫ����H3N+CH2COOH���ݴ�д�����ӷ���ʽΪ: H3N+CH2COOH+2OH��= H2NCH2COO��+2H2O��
�𰸣�H3N+CH2COOH+2OH��= H2NCH2COO��+2H2O
(3)��K=![]() kPa-1�����ñ����е�һ�����ݿ�֪���輡�쵰����Ũ��c
kPa-1�����ñ����е�һ�����ݿ�֪���輡�쵰����Ũ��c
![]()
c����1-50%�� 0.50kPa c��50%
K=![]() =2
=2
�����â��м�������K=2���㼴�ɣ�
2=![]() ������=97.7��
������=97.7��
�𰸣�97.7
�۸��ݱ����ṩ���ݿ�֪p(O2)ԽС����϶�Խ�ͣ� MbO2Ũ��Խ�ͣ�
�𰸣���
�����â��м����K=2������ƽ��ʱv��= v�����м��㣻
ƽ��ʱv��= v���� ��k����c(Mb)��p(O2)=k����c(MbO2)����K��=![]() = k����K=60s-1��2kPa-1=120s-1 KPa-1��
= k����K=60s-1��2kPa-1=120s-1 KPa-1��
��: 120s-1 KPa-1

����Ŀ������Դ�����ĺ��IJ���������ӵ�أ�������������ﮣ�LiFePO4�����缫���ϡ���LiFePO4�Ͼɵ缫��������Al��ʯī�ۣ����ղ���øߴ�Li2CO3�Ĺ�ҵ����ͼ���£�

���ϣ�̼�����ˮ���ܽ�ȣ�
�¶�/�� | 0 | 20 | 40 | 60 | 80 | 100 |
�ܽ��/g | 1.54 | 1.33 | 1.17 | 1.01 | 0.85 | 0.72 |
��1������i��ĥ�����Ŀ����_______��
��2������ii��������NaOH��Һ��������______��
��3������iii���ò�ͬ�������ֱ����ʵ�飬������Li����Ϊ3.7%��ԭ�ϣ�����pHΪ3.5����ȡ1.5h��ʵ�������±���ʾ��
��� | �� | ������ | ����ҺLi+Ũ��(g/L) | ������Li����/% |
ʵ��1 | HCl | H2O2 | 9.02 | 0.10 |
ʵ��2 | HCl | NaClO3 | 9.05 | 0.08 |
ʵ��3 | HCl | O2 | 7.05 | 0.93 |
��ʵ��2�У�NaClO3�����ᷴӦ���ɻ���ɫ���壬���������������������������÷�Ӧ�����ӷ���ʽΪ______��
�ڽ��ʵ�����͢��е���������ѡ��H2O2��Ϊ��������ԭ����______��
�۹���iii�õ��Ľ���Һѭ�����ε�Ŀ����_____��
��4������Һ�д��ڴ���H2PO4��HPO42����֪��H2PO4 HPO42 +H+��HPO42 PO43+H+�����ƽ���ƶ�ԭ����������iv�õ������������ԭ��_____��
��5���Աȹ���iv��v��˵������iv���ñ���Na2CO3��Һ��ԭ��______��
��6����������vi�IJ���_______��