题目内容
【题目】KI广泛应用于分析试剂、感光材料、制药和食品添加剂等。实验室制备KI的装置如下图所示。

已知:①3I2+6KOH![]() 5KI+KIO3+3H2O②3H2S+KIO3
5KI+KIO3+3H2O②3H2S+KIO3![]() KI+3S↓+3H2O
KI+3S↓+3H2O
(1)利用上图装置制备KI,其连接顺序为_____________(按气流方向,用小写字母表示)。
(2)检查装置A气密性的方法是____________;装置D的作用是____________________。
(3)制备KI时,向三颈瓶中逐滴滴入KOH溶液,加热并不断搅拌,观察到棕黄色溶液变为无色时,立即停止滴加KOH溶液,然后通入足量H2S气体。
①反应结束后,向三颈瓶中加入硫酸溶液并加热,可以除去KI溶液中的H2S,原因是________________________________________。
②用肼(N2H4)替代H2S,制得产品纯度更高,理由是_______________(用化学方程式表示)。
(4)设计实验方案除去KI溶液中的稀硫酸_____________________________。
(5)若得到1.6g硫单质,理论上制得KI的质量为_________________g。
【答案】a→e→f→c→d→b 关闭活塞,向球形漏斗内加水至形成一段水柱,一段时间内液柱高度不发生变化,说明装置气密性良好 除去H2S中的HCl气体 加热使H2S的溶解度减小而放出;硫酸电离出的氢离子增大了c(H+),促使H2S电离平衡左移,导致H2S放出。 3N2H4+2KIO3=2KI+3N2↑+6H2O 向KI溶液中加入足量BaCO3固体,充分搅拌后过滤、洗涤,将滤液和洗涤液合并 16.6
【解析】
根据题干信息可知①3I2+6KOH![]() 5KI+KIO3+3H2O②3H2S+KIO3
5KI+KIO3+3H2O②3H2S+KIO3![]() KI+3S↓+3H2O,需要的反应物为H2S、KOH、I2,A装置根据强酸制弱酸原理制备硫化氢气体,FeS+2HCl=FeCl2+H2S↑;D装置用于除去H2S中的HCl气体,导管e进f出;C装置是制取KI的装置,硫化氢气体从c进入装置与其他反应物充分接触,剩余气体从d出去进入B装置,除掉未反应的硫化氢气体防止污染环境。
KI+3S↓+3H2O,需要的反应物为H2S、KOH、I2,A装置根据强酸制弱酸原理制备硫化氢气体,FeS+2HCl=FeCl2+H2S↑;D装置用于除去H2S中的HCl气体,导管e进f出;C装置是制取KI的装置,硫化氢气体从c进入装置与其他反应物充分接触,剩余气体从d出去进入B装置,除掉未反应的硫化氢气体防止污染环境。
(1)根据上面分析可知,制备KI,按气流方向其连接顺序为a→e→f→c→d→b;
答案:a→e→f→c→d→b
(2)装置A是启普发生器,检验气密性可利用压强差原理,方法是关闭活塞,向球形漏斗内加水至形成一段水柱,一段时间内液柱高度不发生变化,说明装置气密性良好;因为盐酸易挥发,所以制得的硫化氢中混有氯化氢,装置D的作用是除去H2S中的HCl气体;
答案:关闭活塞,向球形漏斗内加水至形成一段水柱,一段时间内液柱高度不发生变化,说明装置气密性良好 除去H2S中的HCl气体
(3)制备KI时,向三颈瓶中逐滴滴入KOH溶液,加热并不断搅拌,观察到棕黄色溶液变为无色时,也就是反应3I2+6KOH![]() 5KI+KIO3+3H2O结束,立即停止滴加KOH溶液,然后通入足量H2S气体,发生反应3H2S+KIO3
5KI+KIO3+3H2O结束,立即停止滴加KOH溶液,然后通入足量H2S气体,发生反应3H2S+KIO3![]() KI+3S↓+3H2O。
KI+3S↓+3H2O。
①反应结束后,向三颈瓶中加入硫酸溶液并加热,可以除去KI溶液中的H2S,原因可以从电离平衡和气体溶解度随温度升高而减小分析;
答案:加热使H2S的溶解度减小而放出;硫酸电离出的氢离子增大了c(H+),促使H2S电离平衡左移,导致H2S放出。
②因为肼(N2H4)也具有强还原性,可以肼(N2H4)替代H2S,肼(N2H4)的氧化产物为氮气,可以使制得产品纯度更高,用化学方程式表示为3N2H4+2KIO3=2KI+3N2↑+6H2O。
答案:3N2H4+2KIO3=2KI+3N2↑+6H2O
(4)选择的药品在除杂的同时,要保证不掺入新的杂质,因此选择BaCO3;
答案:向KI溶液中加入足量BaCO3固体,充分搅拌后过滤、洗涤,将滤液和洗涤液合并
(5)根据题干信息①3I2+6KOH![]() 5KI+KIO3+3H2O②3H2S+KIO3
5KI+KIO3+3H2O②3H2S+KIO3![]() KI+3S↓+3H2O,列关系式计算;
KI+3S↓+3H2O,列关系式计算;
6KI ~ 3S
6mol 3mol
n(KI )mol ![]() mol
mol
得n(KI)=0.1mol
m(KI)=n(KI) ×M(KI)=0.1mol×166g/mol=16.6g;
答案:16.6

【题目】含氮化合物与生产、生活、生命和环境息息相关。
(1)NO加速臭氧层被破坏,其反应过程如图所示。
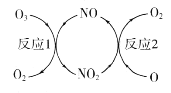
①NO的作用是________________________。
②已知:O3(g)+O(g) =2O2(g) △H=-143kJ·mol-1
反应l:O3(g)+NO(g) =NO2(g)+O2(g)△H1=-200.2kJ·mol-1
则反应2的热化学方程式为____________________________。
(2)肌红蛋白(Mb)是肌肉内储存氧的蛋白质,构成肌红蛋白的甘氨酸(H2NCH2COOH)是一种两性物质,在溶液中以三种离子形式存在,其转化关系如下 三种离子的物质的量分数(δ)与[
三种离子的物质的量分数(δ)与[![]() ]的关系如图所示。
]的关系如图所示。
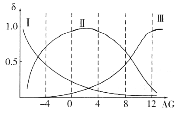
①溶液呈中性时,三种离子浓度由大到小的顺序为______________________。
②向AG=12的溶液中加入过量NaOH溶液时,主要反应的离子方程式为______________。
(3)肌红蛋白(Mb)可与O2结合生成MbO2:![]() 。37℃时测得肌红蛋白的结合度(α)与p(O2)的关系如下表。
。37℃时测得肌红蛋白的结合度(α)与p(O2)的关系如下表。
[结合度(α)指已与O2结合的肌红蛋白占总肌红蛋白的物质的量分数]
p(O2)/kPa | 0.50 | 1.00 | 2.00 | 3.00 | 4.00 | 5.00 | 6.00 |
α(MbO2)% | 50.0 | 66.7 | 80.0 | 85.7 | 88.9 | 90.9 | 92.3 |
①计算37℃时,上述反应的平衡常数K=__________kPa-1(气体和溶液中的溶质分别用分压和物质的量浓度表示)。
②37℃时,若空气中氧气分压为21.0 kPa,则人正常呼吸时α的最大值为________%(保留1位小数)。
③温度不变时,游客在山顶时体内MbO2的浓度比在山下________(填“高”或“低”)。
④研究发现,v正=k正·c(Mb)·p(O




