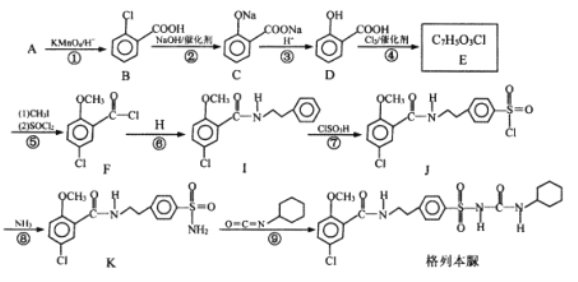
��Ŀ����
����Ŀ��������NOx��SO2����Ⱦ�����Чȥ������Դ�ij�������ǵ���������Ҫ�о����⣬Ŀǰ���õķ������£�
I.ֱ��ת���������ø�Ч����������β��ֱ��ת��Ϊ�����ʡ�
��֪��N2(g)��O2(g)��2NO(g)��H1����180kJ��mol��1
(1)���ù����������ս�NO�ֽ�ΪN2��O2��������Ⱦ��
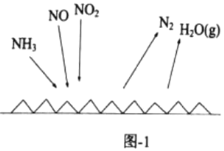
��![]() �ֱ��ʾN2��NO��O2����������ڹ����������ֽ�NO�Ĺ�����ͼ��ʾ���������������Ĺ����У�����״̬��͵���________________________(����ĸ���)��
�ֱ��ʾN2��NO��O2����������ڹ����������ֽ�NO�Ĺ�����ͼ��ʾ���������������Ĺ����У�����״̬��͵���________________________(����ĸ���)��
![]()
(2)����ϡ���ȴ����ܽ�����β���е�CO��NOֱ��ת����������N2��CO2��
����֪��C(s)��CO(g)��ȼ���ȷֱ�Ϊ393kJ��mol��1��283kJ��mol��1��д��NO(g)��CO(g)��ת����N2(g)��CO2(g)���Ȼ�ѧ����ʽ________________________��
��Ϊ�о�������Ӧ��ijѧϰС�����ܱ������г���10molCO��10molNO��������������д��Ӧ��ʵ����ƽ��ʱNO������������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��

a.ij�¶��µ�ƽ��״̬D�㣬��ͬʱ��ȡ��С��������ͽ����¶ȣ����´ﵽƽ��״̬ʱ�����ܵ���ͼ��A��G���е�________________________�㡣
b.��ѹǿΪ10MPa���¶�ΪTʱ��ƽ�ⳣ��Kp��________________________(��ƽ���ѹ����ƽ��Ũ�ȼ��㣬��ѹ����ѹ�����ʵ�������������3λ��Ч����)��
II.����ԭ�������û�ԭ���ڴ����������½���������תΪ������N2��CO2��
(3)�û���̿��ԭ�����Դ�������β���еĵ������
ij�о�С����2L�ĺ����ܱ������м���һ������NO�������Ĺ������̿��������Ӧ��C(s)��2NO(g)![]() N2(g)��CO2(g)���ڲ�ͬ�¶��²��ƽ����ϵ�и����ʵ����ʵ��������
N2(g)��CO2(g)���ڲ�ͬ�¶��²��ƽ����ϵ�и����ʵ����ʵ��������
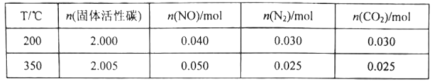
�ٸ÷�Ӧ������ӦΪ________________________(����ȡ����ȡ�)��Ӧ��
��350��ʱ����Ӧ�ﵽƽ���������������ٳ���0.100molNO���ٴδﵽƽ���N2���������ӦΪ________________________��
A.0.5B.0.25C.����0.25��0.5֮��D.��ȷ��
III.��ⷨ�����õ��ķ�������������ת��Ϊ�������ʣ��Ӷ��ﵽ��Դ�Ļ������á�
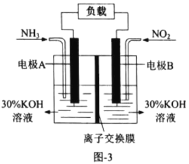
(4)��ͼ��ʾ�ĵ��װ�ã��ɽ������е�NO2��SO2ת��Ϊ����泥��Ӷ�ʵ�ַ����Ļ��������ã��ش��������⣺
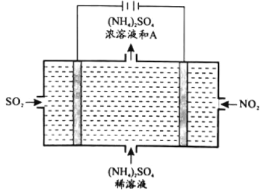
�������ĵ缫��ӦʽΪ________________________��
����ͼ��A������________________________��
���𰸡�C 2NO(g)��2CO(g)===N2(g)��2CO2(g)��H����746kJ��mol��1 G 0.474MPa��1 ���� B NO2��8H+��7e��===![]() ��2H2O H2SO4������
��2H2O H2SO4������
��������
���ݻ�ѧ��Ӧ�м��Ķ������γɹ����������仯��ʽ�жϣ����ݸ�˹���ɷ����жϣ��������ܱ������г���10molCO��10molNO��������Ӧ��ѹǿΪ10MPa���¶�ΪT1�£�����Ӧ���е�20min�ﵽƽ��״̬��NO�������Ϊ25%��������м�����ʽ���㣻����¶ȶ�ƽ���Ӱ����������ݵ��ԭ�������жϣ����е��ص�����������ԭ��Ӧ��
I��(1)��ѧ��Ӧ�����д��ڼ��Ķ��Ѻͼ����γɣ����ж��ѻ�ѧ�����ȣ��γɻ�ѧ���ų���������NO�ֽ�ΪN2��O2�Ĺ����У�C�������γɻ�ѧ�����ͷ����������ʱ����״̬��ͣ�
(2) ����֪����N2(g)��O2(g)��2NO(g)��H1����180kJ��mol��1��C(s)��CO(g)��ȼ���ȷֱ�Ϊ393kJ��mol��1��283kJ��mol��1����C(s)+ O2(g)��CO2(g) ��H2=-393kJ��mol��1����CO(g) + ![]() O2(g)��CO2(g) ��H3=-283kJ��mol��1�����ݸ�˹���ɿ�֪����2-�ٿɵ�2NO(g)��2CO(g)===N2(g)��2CO2(g)���˷�Ӧ����H��(-283kJ��mol��1)��2-(��180kJ��mol��1)=��746kJ��mol��1����NO(g)��CO(g)��ת����N2(g)��CO2(g)���Ȼ�ѧ����ʽΪ2NO(g)��2CO(g)===N2(g)��2CO2(g)��H����746kJ��mol��1��
O2(g)��CO2(g) ��H3=-283kJ��mol��1�����ݸ�˹���ɿ�֪����2-�ٿɵ�2NO(g)��2CO(g)===N2(g)��2CO2(g)���˷�Ӧ����H��(-283kJ��mol��1)��2-(��180kJ��mol��1)=��746kJ��mol��1����NO(g)��CO(g)��ת����N2(g)��CO2(g)���Ȼ�ѧ����ʽΪ2NO(g)��2CO(g)===N2(g)��2CO2(g)��H����746kJ��mol��1��
��a����֪2NO(g)��2CO(g)===N2(g)��2CO2(g)��H����746kJ��mol��1����С���������������ѹǿ��ƽ��������Ӧ�����ƶ��������¶ȣ�ƽ�������ƶ���ƽ����ϵ��NO������������ͣ����´ﵽƽ��״̬ʱ��������ͼ��G�㣻
b����ѹǿΪ10MPa���¶�ΪT1ƽ��ʱNO���������Ϊ25%��
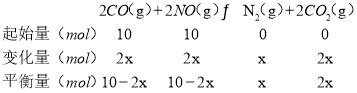
![]() ��100%=25%����ã�x=
��100%=25%����ã�x=![]() mol��ƽ��������������ʵ���Ϊ20-x=
mol��ƽ��������������ʵ���Ϊ20-x=![]() mol�����¶��µ�ƽ�ⳣ��Kp��
mol�����¶��µ�ƽ�ⳣ��Kp��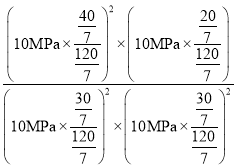 =0.474MPa��1��
=0.474MPa��1��
II��(3)���ɱ������ݿ�֪�����¶�ʣ��NO�϶࣬˵�������¶����Ʒ�Ӧ�������ƶ���������ӦΪ���ȷ�Ӧ��
��350��ʱ��ԭƽ����ϵ��N2���������Ϊ![]() =25%���Է�ӦC(s)+2NO(g)N2(g)+CO2(g)�����ڷ�Ӧǰ������ķ��������䣬������ѹǿƽ�ⲻ�ƶ�����Ӧ�ﵽƽ���������������ٳ���0.100 mol NO���൱������ѹǿ����ƽ�ⲻ�ƶ����������NO�İٷֺ������䣬����Ϊ25%���ʴ�ΪB��
=25%���Է�ӦC(s)+2NO(g)N2(g)+CO2(g)�����ڷ�Ӧǰ������ķ��������䣬������ѹǿƽ�ⲻ�ƶ�����Ӧ�ﵽƽ���������������ٳ���0.100 mol NO���൱������ѹǿ����ƽ�ⲻ�ƶ����������NO�İٷֺ������䣬����Ϊ25%���ʴ�ΪB��
III��(4)�����Դ���������ļ�Ϊ������NO2��������������ԭ��Ӧ����NH4+����缫��ӦʽΪNO2��8H+��7e��===![]() ��2H2O��
��2H2O��
��SO2�ڵ��ص��������淢��������Ӧ���缫��ӦʽΪSO2-2e-+2H2O=SO42-+4H+���ܷ�ӦʽΪ
2NO2+10H2O+7SO2=(NH4)2SO4��6H2SO4

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ������ʵ�����������ͽ��۾���ȷ���ǣ� ��
ѡ�� | ʵ����� | ʵ������ | ���� |
A | ��ij�л�����������Ȼ�̼��Һ��� | ��Һ��ɫ | ���л����бض�����̼̼˫�� |
B | ������������Һ�еμ����ᱵ��ϡ���� | ������ɫ���� | ����������Һ�ѱ��� |
C | �����Ը��������Һ�еμ�˫��ˮ | ��ɫ��ȥ���������� | H2O2���л�ԭ�� |
D | ���Ҵ���Һ�м�һС���� | �������� | �Ҵ������ǻ� |
A.AB.BC.CD.D