��Ŀ����
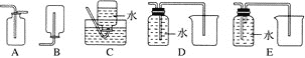
����Ŀ��������ͼ��ʾװ�òⶨ�к��ȵ�ʵ�鲽�����£�

������Ͳ��ȡ50mL 0.50molL-1���ᵹ��С�ձ��У����������¶ȣ�Ȼ����¶ȼ��ϵ� ����ˮ��ϴ�ɾ���
������һ��Ͳ��ȡ50mL 0.55molL-1 NaOH��Һ������ͬһ�¶ȼƲ�����¶ȣ�
�۽�NaOH��Һ����С�ձ��У��跨ʹ֮��Ͼ��ȣ���û��Һ����¶ȡ��ش��������⣺
��1��ʵ����NaOH��Һ���Թ�����Ŀ����_________________________________________��
��2��ʹ������NaOH��Һ��Ͼ��ȵ���ȷ������_______������ţ���
A�����¶ȼ�С�Ľ��� B���ҿ�ӲֽƬ�ò���������
C����������ձ� D���������¶ȼ��ϵĻ��β����������������½���
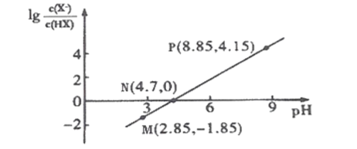
��3��ʵ���и���60mL 0.50mol��L-1������50mL 0.55mol��L-1 NaOH��Һ���з�Ӧ��������ʵ����ȣ��ų�������______________(���� ƫ������ƫ�����������������ͬ)������õ��к��Ȧ�H ______________��
��4���������ʵ�����к��Ȧ�H = -55.6kJ/mol������֪��Ba2+(aq)+2OH-(aq)+ 2H++SO42- (aq)=BaSO4(s) +2H2O(l) ����H��-1584.2 kJ��mol-1��������BaSO4(s)�ķ�Ӧ����H��______________��
���𰸡�ʹ������ȫ�к� D ƫ�� ��� -1473 kJ/mol
��������
��1��Ϊ��ȷ�����������ᷴӦ��ȫ������NaOH�Թ�����
��2�����������������Һ���ʱ���������¶ȼ��ϵĻ��β����������������½�����ʹ������NaOH��Һ��Ͼ��ȣ�
��3����Ӧ�ų����������������Լ�������Ķ����йأ����к�����ָǿ�ᡢǿ���ϡ��Һ��Ӧ����1molˮ����Ӧ�ķ�Ӧ�ȣ������������أ��������к��ȵĸ����ʵ�����ش�
��4������Ϣ��֪��
��Ba2+(aq)+2OH-(aq)+ 2H++SO42- (aq)=BaSO4(s) +2H2O(l) ��H��-1584.2 kJ��mol-1
��OH-(aq)+ H+ (aq)= H2O(l) ��H��-55.6 kJ��mol-1
�ɸ�˹���ɿ�֪����-����2�õ�Ba2+��aq)+SO42- (aq)=BaSO4(s)�Ħ�H���ݴ˽��
��1��ʵ��NaOH��Һ���Թ�����Ŀ����ȷ�����������ᱻ��ȫ�кͣ��������������������ˮ�����ʵ������������㷴Ӧ�ȣ�
�ʴ�Ϊ��ʹ������ȫ�кͣ�
��2��ʹ������NaOH��Һ��Ͼ��ȵ���ȷ���������ǣ��������¶ȼ��ϵĻ��β����������������½�����
�ʴ�Ϊ��D��
��3������кͷ�ӦΪ���ȷ�Ӧ������ˮԽ�࣬��Ӧ�ų�������Խ�࣬���ʵ���и���60mL0.50molL-1��������50mL0.55molL-1��NaOH��Һ���з�Ӧ��������ʵ����ȣ�����ˮ�����ʵ������ӣ����ų�������ƫ�ࣻ���к�����ָǿ�ᡢǿ���ϡ��Һ��Ӧ����1molˮ����Ӧ�ķ�Ӧ�ȣ���������к�����ȣ�
�ʴ�Ϊ��ƫ�ࣻ��ȣ�
��4������Ϣ��֪��
��Ba2+(aq)+2OH-(aq)+ 2H++SO42- (aq)=BaSO4(s) +2H2O(l) ��H��-1584.2 kJ��mol-1
��OH-(aq)+ H+ (aq)=H2O(l) ��H��-55.6 kJ��mol-1
�ɸ�˹���ɿ�֪����-����2�õ�Ba2+��aq)+SO42- (aq)=BaSO4(s)��
�䷴Ӧ�Ȧ�H=-1584.2 kJ��mol-1(-55.6 kJ��mol-1)��2=1473 kJmol1��
�ʴ�Ϊ��-1473 kJ/mol��

 һ���㶨ϵ�д�
һ���㶨ϵ�д� ��У��ҵ��ϵ�д�
��У��ҵ��ϵ�д� ���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
���ɶ���ܲ��¿�ֱͨ��Уϵ�д�����Ŀ��T��ʱ����2L���ܱ������У�����X��Y��Z�����ʵ�����ʱ��ı仯������ͼ��ʾ��
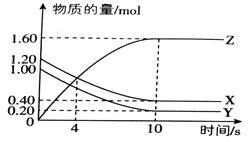
��1���÷�Ӧ�Ļ�ѧ����ʽΪ_________________________.
��2��0~10s�ڣ�X�Ļ�ѧ��Ӧ����Ϊ___________________.
��3���÷�Ӧ�ڲ�ͬ�¶��µ�ƽ�ⳣ�������ʾ��
T/�� | 100 | 220 | 830 | 1000 | 1200 |
K | 45.00 | 32.00 | 1.00 | 0.60 | 0.38 |
��÷�Ӧ�Ħ�H__________0 (�>������<����=��)
��4��830��ʱ�����ݻ�Ϊ10L�ĺ����ܱ������г���5molX���塢7.8molY�����7.1mol Z���壬��ʱ��(��)_______��(��) (�>������<����=��)
��5����ͼ��ʾ��Ӧ���¶�Ϊ_________________��
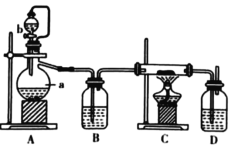
����Ŀ����ͼ��ʵ�����Ʊ�����������һϵ�����ʵ���װ�ã��гּ������������ԣ���
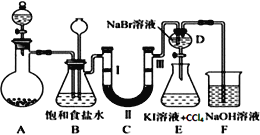
��1��װ��B�б���ʳ��ˮ��������__��ͬʱװ��B���ǰ�ȫƿ�����ʵ�����ʱC���Ƿ�����������д����������ʱB�е�����___��
��2��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C�Т����η���___��������ţ�
a | b | c | d | |
�� | �������ɫ���� | �������ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
�� | ��ʯ�� | �轺 | Ũ���� | ��ˮ�Ȼ��� |
�� | ʪ�����ɫ���� | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
��4����ҵ�Ͻ�����ͨ��ʯ������ȡƯ�ۣ���ѧ��Ӧ����ʽΪ__��Ư�۳����ڿ����л�ʧЧ����д��ʧЧ��������Ӧ�Ļ�ѧ����ʽ___��