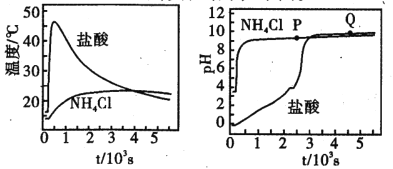
ΧβΡΩΡΎ»ί
ΓΨΧβΡΩΓΩTΓφ ±Θ§‘Ύ2LΒΡΟή±’»ίΤς÷–Θ§ΤχΧεXΓΔYΓΔZΒΡΈο÷ ΒΡΝΩΥφ ±ΦδΒΡ±δΜ·«ζœΏ»γΆΦΥυ ΨΓΘ

Θ®1Θ©ΗΟΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣ_________________________.
Θ®2Θ©0~10sΡΎΘ§XΒΡΜ·―ßΖ¥”ΠΥΌ¬ ΈΣ___________________.
Θ®3Θ©ΗΟΖ¥”Π‘Ύ≤ΜΆ§Έ¬Ε»œ¬ΒΡΤΫΚβ≥Θ ΐ»γ±μΥυ ΨΘΚ
T/Γφ | 100 | 220 | 830 | 1000 | 1200 |
K | 45.00 | 32.00 | 1.00 | 0.60 | 0.38 |
‘ρΗΟΖ¥”ΠΒΡΠΛH__________0 (ΧνΓΑ>Γ±ΓΔΓΑ<Γ±ΜρΓΑ=Γ±)
Θ®4Θ©830Γφ ±Θ§‘Ύ»ίΜΐΈΣ10LΒΡΚψ»ίΟή±’»ίΤς÷–≥δ»κ5molXΤχΧεΓΔ7.8molYΤχΧεΚΆ7.1mol ZΤχΧεΘ§¥Υ ±Π‘(’ΐ)_______Π‘(Ρφ) (ΧνΓΑ>Γ±ΓΔΓΑ<Γ±ΜρΓΑ=Γ±)
Θ®5Θ©…œΆΦΥυ ΨΖ¥”ΠΒΡΈ¬Ε»ΈΣ_________________ΓΘ
ΓΨ¥πΑΗΓΩX + Y ![]() 2 Z 0.04 mol/(LΓΛs) < < 220Γφ
2 Z 0.04 mol/(LΓΛs) < < 220Γφ
ΓΨΫβΈωΓΩ
Θ®1Θ©ΗυΨίΜ·―ßΖ¥”Π÷–ΗςΈο÷ ΒΡΈο÷ ΒΡΝΩΒΡ±δΜ·ΝΩ”κΜ·―ßΦΤΝΩ ΐ÷°±»≥ ’ΐ±» ι–¥Μ·―ßΖΫ≥Χ ΫΘΜ
Θ®2Θ©ΗυΨίΙΪ ΫΠ‘=![]() ΦΤΥψΜ·―ßΖ¥”ΠΥΌ¬ ΘΜ
ΦΤΥψΜ·―ßΖ¥”ΠΥΌ¬ ΘΜ
Θ®3Θ©”…±μΗώ÷– ΐΨίΩ…÷ΣΘ§Έ¬Ε»…ΐΗΏΘ§ΤΫΚβ≥Θ ΐΦθ–ΓΘ§Φ¥…ΐΈ¬ΤΫΚβΡφœρ“ΤΕ·Θ§Εχ…ΐΈ¬ΤΫΚβœρΈϋ»»Ζ¥”ΠΖΫœρ“ΤΕ·Θ§‘ρΡφΖ¥”ΠΈΣΈϋ»»Ζ¥”ΠΘ§’ΐΖ¥”ΠΈΣΖ≈»»Ζ¥”ΠΘΜ
Θ®4Θ©ΦΤΥψ¥ΥΩΧΗΟΖ¥”ΠΒΡ≈®Ε»…ΧΘ§≤Δ”κΗΟΈ¬Ε»œ¬ΒΡΤΫΚβ≥Θ ΐΫχ––±»ΫœΘ§Ψί¥Υ≈–ΕœΖ¥”ΠΫχ––ΒΡΖΫœρΘ§ΫχΕχ±»Ϋœ’ΐΡφΖ¥”ΠΥΌ¬ ΒΡ¥σ–ΓΘΜ
Θ®5Θ©ΗυΨίΆΦ ΨΦΤΥψΤΫΚβ ±ΗςΈο÷ ΒΡ≈®Ε»Θ§ΦΤΥψΤΫΚβ≥Θ ΐΘ§≈–ΕœΥυ¥ΠΒΡΈ¬Ε»ΓΘ
Θ®1Θ©”…ΆΦœσΩ…“‘Ω¥≥ωXΓΔYΒΡΈο÷ ΒΡΝΩΦθ–ΓΘ§ZΒΡΈο÷ ΒΡΝΩ‘ωΕύΘ§‘ρXΓΔYΈΣΖ¥”ΠΈοΘ§ZΈΣ…ζ≥…ΈοΘ§Μ·―ßΖ¥”Π÷–ΗςΈο÷ ΒΡΈο÷ ΒΡΝΩΒΡ±δΜ·ΝΩ”κΜ·―ßΦΤΝΩ ΐ÷°±»≥ ’ΐ±»Θ§‘ρ”–![]() n(X):
n(X):![]() n(Y):
n(Y):![]() n(Z)=(1.20mol0.40mol):(1.00mol0.20mol):1.60mol=1:1:2Θ§‘ρΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣX+Y2ZΘΜ
n(Z)=(1.20mol0.40mol):(1.00mol0.20mol):1.60mol=1:1:2Θ§‘ρΖ¥”ΠΒΡΜ·―ßΖΫ≥Χ ΫΈΣX+Y2ZΘΜ
Ι ¥πΑΗΈΣΘΚX+Y2ZΘΜ
Θ®2Θ©0~10sΡΎΘ§XΒΡΜ·―ßΖ¥”ΠΥΌ¬ ΈΣΠ‘Θ®XΘ©=Θ®1.20mol-0.40molΘ©Γ¬2LΓ¬10s=0.04 mol/(LΓΛs)ΘΜ
Ι ¥πΑΗΈΣΘΚ0.04 mol/(LΓΛs)ΘΜ
Θ®3Θ©”…±μΗώ÷– ΐΨίΩ…÷ΣΘ§Έ¬Ε»…ΐΗΏΘ§ΤΫΚβ≥Θ ΐΦθ–ΓΘ§Φ¥…ΐΈ¬ΤΫΚβΡφœρ“ΤΕ·Θ§Εχ…ΐΈ¬ΤΫΚβœρΈϋ»»Ζ¥”ΠΖΫœρ“ΤΕ·Θ§‘ρΡφΖ¥”ΠΈΣΈϋ»»Ζ¥”ΠΘ§’ΐΖ¥”ΠΈΣΖ≈»»Ζ¥”ΠΘ§‘ρΗΟΖ¥”ΠΒΡΠΛH<0ΘΜ
Ι ¥πΑΗΈΣΘΚ<ΘΜ
Θ®4Θ©830Γφ ±Θ§ΗΟΖ¥”ΠΒΡΤΫΚβ≥Θ ΐΈΣ1.00Θ§¥ΥΩΧΗΟΖ¥”ΠΒΡ≈®Ε»…ΧΈΣ![]() Θ§‘ρΖ¥”ΠΫΪœρΡφΖ¥”ΠΖΫœρΫχ––Θ§Π‘(’ΐ)<Π‘(Ρφ)ΘΜ
Θ§‘ρΖ¥”ΠΫΪœρΡφΖ¥”ΠΖΫœρΫχ––Θ§Π‘(’ΐ)<Π‘(Ρφ)ΘΜ
Ι ¥πΑΗΈΣΘΚ<ΘΜ
Θ®5Θ©…œΆΦΥυ ΨΘ§¥οΒΫΤΫΚβ ±XΓΔYΓΔZΒΡΈο÷ ΒΡΝΩΖ÷±πΈΣ0.40molΓΔ0.20molΓΔ1.60molΘ§»ίΤς»ίΜΐΈΣ2LΘ§≈®Ε»Ζ÷±πΈΣ0.20 mol/LΓΔ0.10 mol/LΓΔ0.80 mol/LΘ§‘ρΤΫΚβ≥Θ ΐΈΣ![]() Θ§‘ρΈ¬Ε»ΈΣ220ΓφΘΜ
Θ§‘ρΈ¬Ε»ΈΣ220ΓφΘΜ
Ι ¥πΑΗΈΣΘΚ220ΓφΓΘ

ΓΨΧβΡΩΓΩH2O2ΉςΈΣ¬Χ…Ϊ―θΜ·ΦΝ±Μ”Π”Ο”ΎΖœΥ°¥ΠάμΓΔ‘λ÷ΫΚΆΜ·―ßΚœ≥…Β»––“ΒΓΘ
Θ®1Θ©“―÷ΣΘΚH2(g)+![]() O2(g)=H2O(l) ΠΛH1=Θ≠285.8kJΓΛmol-1
O2(g)=H2O(l) ΠΛH1=Θ≠285.8kJΓΛmol-1
H2(g)+O2(g)=H2O2(l) ΠΛH2=Θ≠135.8kJΓΛmol-1
ΔΌH2(g)”κO2(g)ΒΡΖ¥”Π÷–Θ§‘Ύ»»ΝΠ―ß…œΗϋ”–άϊΒΡ≤ζΈο «__Θ§‘≠“ρ «__ΓΘ
ΔΎ≥ΘΈ¬œ¬Θ§H2O2Ζ÷ΫβΒΡ»»Μ·―ßΖΫ≥Χ ΫΈΣ__ΓΘ
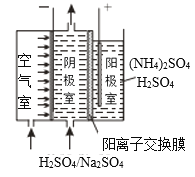
Θ®2Θ©Έ“ΙζΩΤ―ßΦ“ Ι”ΟAg9Ά≈¥ΊΉς¥ΏΜ·ΦΝΘ§―–ΨΩH2O2ΒΡΚœ≥…ΓΘΗς≤Ϋ÷ηΒΡΜνΜ·ΡήΚΆΖ¥”Π»»Θ§»γ±μΥυ ΨΘ§άϊ”ΟΦΤΥψΜζΡΘΡβΖ¥”Πάζ≥Χ»γΆΦΥυ ΨΘ®TS±μ ΨΙΐΕ…Χ§Θ§±μ Ψ±Μ¥ΏΜ·ΦΝΈϋΗΫΒΡΈο÷÷Θ©ΓΘ
Ag9Ά≈¥Ί…œ…ζ≥…H2O2ΒΡΜνΜ·ΡήEaΚΆΖ¥”Π»»![]()
≤Ϋ÷η | ΙΐΕ…Χ§ | Ea/kJ |
| |
A | Ag9 | TS1 | 74.1 | +68.7 |
B | HΓΣAg9 | TS2 | 108.7 | -27.2 |
C | HΓΣAg9ΓΣH+ O2 | TS3 | 78.4 | -75.4 |
D | HOO | TS4 | 124.7 | +31.3 |

ΔΌΆ®ΙΐΫΒΒΆ≤Ϋ÷η___Θ®ΧνΉ÷ΡΗΘ©ΒΡΡήάίΘ®ΜνΜ·ΡήΘ©Θ§Ω…“‘Ϋœ¥σΖυΕ»ΧαΗΏΚœ≥…Ζ¥”ΠΒΡΥΌ¬ ΓΘ
ΔΎΖ¥”Πάζ≥Χ÷–2ΒΫ3ΕœΝ―ΒΡΜ·―ßΦϋΈΣ___Θ®Χν–ρΚ≈Θ©ΓΘ
A.O2÷–ΒΡ―θ―θΦϋ B.H2÷–ΒΡ«β«βΦϋ C.Ag9OOH÷–ΒΡ―θ«βΦϋ
Θ®3Θ©άϊ”Ο“θ―τΦΪΆ§≤ΫΖ≈Βγ≤ζ…ζH2O2ΚΆΙΐΝρΥαοß[(NH4)2S2O8]ΒΡ‘≠άμ»γΆΦΥυ ΨΓΘ―τΦΪ…œΖ≈ΒγΒΡάκΉ” «___Θ§“θΦΪΒΡΒγΦΪΖ¥”Π ΫΈΣ___ΓΘ
Θ®4Θ©≥ΘΈ¬œ¬Θ§H2O2Ζ÷ΫβΥΌ¬ ΖΫ≥Χv=0.0625ΓΛc(H2O2)mgΓΛL-1ΓΛs-1Θ§c(H2O2)Υφ ±Φδ±δΜ·»γœ¬±μΘΚ
C(H2O2) (mg | 10000.0 | 8000.0 | 4000.0 | 2000.0 | 1000.0 |
Ζ÷Ϋβ ±Φδ(s) | 0 | 7 | 23 | 39 | 55 |
ΔΌΒ±c(H2O2)=8000.0mgΓΛL-1 ±Θ§v=__mgΓΛL-1ΓΛs-1ΘΜ
ΔΎΒ±c(H2O2)ΫΒΈΣ5000.0mgΓΛL-1 ±Θ§Ζ÷Ϋβ ±ΦδΈΣ___sΓΘ