��Ŀ����
����Ŀ����ͼ��ʵ�����Ʊ�����������һϵ�����ʵ���װ�ã��гּ������������ԣ���
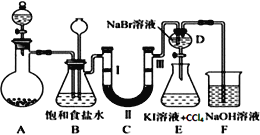
��1��װ��B�б���ʳ��ˮ��������__��ͬʱװ��B���ǰ�ȫƿ�����ʵ�����ʱC���Ƿ�����������д����������ʱB�е�����___��
��2��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C�Т����η���___��������ţ�
a | b | c | d | |
�� | �������ɫ���� | �������ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
�� | ��ʯ�� | �轺 | Ũ���� | ��ˮ�Ȼ��� |
�� | ʪ�����ɫ���� | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
��4����ҵ�Ͻ�����ͨ��ʯ������ȡƯ�ۣ���ѧ��Ӧ����ʽΪ__��Ư�۳����ڿ����л�ʧЧ����д��ʧЧ��������Ӧ�Ļ�ѧ����ʽ___��
���𰸡���ȥCl2�е�HCl B����ƿˮλ�½�������©����Һ���������γ�ˮ�� d 2Ca(OH)2+2Cl2=CaCl2+Ca(ClO)2+2H2O Ca(ClO)2+CO2+H2O = 2HClO+CaCO3��2HClO![]() 2HCl + O2��
2HCl + O2��
��������
��1��ʵ�����ö���������Ũ���������ȡ�����������ӷ�����Ӧ��ȡ�������к����Ȼ��⣬�Ȼ���������ˮ�������ڱ���ʳ��ˮ���ܽ�Ȳ����ñ���ʳ��ˮ��ȥCl2�е�HCl��װ��B���ǰ�ȫƿ�����ʵ�����ʱC���Ƿ�����������������ʱB�е�ѹǿ����B����ƿˮλ�½�������©����Һ���������γ�ˮ����
��2��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ���֤�����Ƿ����Ư���ԣ�Ҫ��֤����������Ư���ԣ�ʪ�����ɫ�����У�������ˮ��Ӧ���ɴ��������Ư���ԣ������Ҫ����������ѡ����abcd�����ж��Ǹ������Ӧʢ�Ź����������ٽ����������ͨ��������ɫ������֤�����Ƿ���Ư���ԣ�
��3����ҵ�Ͻ�����ͨ��ʯ������ȡƯ�ۣ����ɴ�����ƺ��Ȼ��ƣ�Ư�۳��ڷ��ڿ����л�ʧЧ����Ϊ�����к���CO2��Ca(ClO)2��CO2��H2O��Ӧ����HClO��CaCO3�����ɵ�HClO���һ���ֽ����������������
��1��ʵ�����ö���������Ũ���������ȡ�����������ӷ�����Ӧ��ȡ�������к����Ȼ��⣬�Ȼ���������ˮ�������ڱ���ʳ��ˮ���ܽ�Ȳ���Bװ�ÿ��Գ�ȥCl2�е�HCl��װ��B���ǰ�ȫƿ�����ʵ�����ʱC���Ƿ�����������������ʱB�е�ѹǿ����B����ƿˮλ�½�������©����Һ���������γ�ˮ����
�ʴ�Ϊ����ȥCl2��HCl��B����ƿˮλ�½�������©����Һ���������γ�ˮ����
��2����Bװ���е������dz�ʪ��Cl2��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ���֤�����Ƿ����Ư���ԣ�Ҫ��֤����������Ư���ԣ�ʪ�����ɫ�����У�������ˮ��Ӧ���ɴ��������Ư���ԣ�ѡ����abcd�����ж��Ǹ������Ӧʢ�Ź����������ٽ����������ͨ��������ɫ������֤�����Ƿ���Ư���ԣ�����C��I��II��III���η���ʪ�����ɫ��������ˮ�Ȼ��ơ��������ɫ����������ѡd��
�ʴ�Ϊ��d��
��3����ҵ�Ͻ�����ͨ��ʯ������ȡƯ�ۣ���ѧ��Ӧ����ʽΪ��2Ca(OH)2+2Cl2=CaCl2+Ca(ClO)2+2H2O��Ư�۳��ڷ��ڿ����л�ʧЧ����Ϊ�����к���CO2��������Ӧ��Ca(ClO)2+CO2+H2O=2HClO+CaCO3�����ɵ�HClO���һ���ֽ⣬������Ӧ��2HClO![]() 2HCl + O2����
2HCl + O2����
�ʴ�Ϊ��Ca(ClO)2+CO2+H2O=2HClO+CaCO3��2Ca(OH)2+2Cl2=CaCl2+Ca(ClO)2+2H2O��2HClO![]() 2HCl+O2����
2HCl+O2����