ЬтФПФкШн
ЁОЬтФПЁПЯТСаЫЕЗЈе§ШЗЕФЪЧ( )
A.NAДњБэАЂЗќМгЕТТоГЃЪ§ЃЌ1mol C4H10жаКЌЙВМлМќЪ§ФПЮЊ14NA
B.ФГЗДгІЕФІЄH = Ѓ88kJmol-1ЃЌдђе§ЗДгІЛюЛЏФмвЛЖЈаЁгк88kJmol-1
C.вбжЊФГЮТЖШЯТЃЌKw = 1ЁС10-13ЃЌШєНЋpH =8ЕФNaOHШмвКгыpH =5ЕФH2SO4ШмвКЛьКЯБЃГжЮТЖШВЛБфЃЌгћЪЙЛьКЯШмвКpH =7ЃЌдђNaOHШмвКгыH2SO4ШмвКЕФЬхЛ§БШЮЊ11ЃК9
D.НЋХЈЖШЮЊ0.1molL-1 HFШмвКМгЫЎВЛЖЯЯЁЪЭЙ§ГЬжаЃЌЕчРыЖШКЭKa(HF)БЃГжВЛБфЃЌ![]() ЪМжеБЃГждіДѓ
ЪМжеБЃГждіДѓ
ЁОД№АИЁПC
ЁОНтЮіЁП
A. 1mol C4H10жаКЌ3ИіCЁЊCЁЂ10ИіCЁЊHЙВМлМќЃЌдђЙВМлМќЪ§ФПЮЊ13NAЃЌЙЪAДэЮѓЃЛ
B. ьЪБфЕФДѓаЁОіЖЈгке§ФцЗДгІЕФЛюЛЏФмЕФВюжЕЃЌФГЗДгІЕФІЄH = Ѓ88kJmol-1ЃЌдђе§ЗДгІЛюЛЏФмВЛвЛЖЈаЁгк88kJmol-1ЃЌЙЪBДэЮѓЃЛ
C. ЫЎЕФРызгЛ§ГЃЪ§ЮЊ1ЁС10-13ЃЌ![]() ЃЌpH =6.5ЪБЃЌШмвКГЪжаадЃЌpH =5ЕФH2SO4ШмвКжа
ЃЌpH =6.5ЪБЃЌШмвКГЪжаадЃЌpH =5ЕФH2SO4ШмвКжа![]() ЃЌpH =8ЕФNaOHШмвКЃЌИљОн
ЃЌpH =8ЕФNaOHШмвКЃЌИљОн![]() ЃЌ
ЃЌ ЃЌвЊЪЙЛьКЯвКЕФpH =7ЃЌШмвКГЪМюадЃЌМДЧтбѕИљРызгЙ§СПЃЌЛьКЯШмвКжаЧтбѕИљРызгЕФЮяжЪЕФСПХЈЖШ
ЃЌвЊЪЙЛьКЯвКЕФpH =7ЃЌШмвКГЪМюадЃЌМДЧтбѕИљРызгЙ§СПЃЌЛьКЯШмвКжаЧтбѕИљРызгЕФЮяжЪЕФСПХЈЖШ![]() ЃЌЩшСђЫсШмвКЁЂNaOHШмвКЕФЬхЛ§вРДЮЮЊXЁЂYЃЌдђСНжжШмвКЛьКЯКѓЙ§СПOH-ЮяжЪЕФСПХЈЖШЮЊ
ЃЌЩшСђЫсШмвКЁЂNaOHШмвКЕФЬхЛ§вРДЮЮЊXЁЂYЃЌдђСНжжШмвКЛьКЯКѓЙ§СПOH-ЮяжЪЕФСПХЈЖШЮЊ![]() ЃЌЫљвдYЃКX = 11:9ЃЌЙЪCе§ШЗЃЛ
ЃЌЫљвдYЃКX = 11:9ЃЌЙЪCе§ШЗЃЛ
D. НЋХЈЖШЮЊ0.1molL-1 HFШмвКМгЫЎВЛЖЯЯЁЪЭЙ§ГЬжаЃЌЕчРыЖШдіДѓЃЌKa(HF)БЃГжВЛБфЃЌcЃЈH+ЃЉЁЂcЃЈF-ЃЉМѕаЁЃЌcЃЈOH-ЃЉдіДѓЃЌШмвКжаЕФЕчКЩЪиКуc(H+)=c(F-)+c(OH-)ЃЌ![]() =
=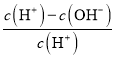 =1-
=1-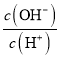 МѕаЁЃЌЙЪDДэЮѓЃЛ
МѕаЁЃЌЙЪDДэЮѓЃЛ
злЩЯЫљЪіЃЌД№АИЮЊCЁЃ

ЁОЬтФПЁПвЛЖЈЮТЖШЯТЃЌЯђ2.0LКуШнУмБеШнЦїжаГфШы2molSO2КЭ1molO2ЃЌЗЂЩњЗДгІЮЊЃК2SO2(g)+O2(g) ![]() 2SO3(g)ЁЃОЙ§вЛЖЮЪБМфКѓДяЕНЦНКтЃЌЗДгІЙ§ГЬжаВтЖЈЕФВПЗжЪ§ОнМћЯТБэЃКЯТСаЫЕЗЈе§ШЗЕФЪЧЃЈ ЃЉ
2SO3(g)ЁЃОЙ§вЛЖЮЪБМфКѓДяЕНЦНКтЃЌЗДгІЙ§ГЬжаВтЖЈЕФВПЗжЪ§ОнМћЯТБэЃКЯТСаЫЕЗЈе§ШЗЕФЪЧЃЈ ЃЉ
t/s | 0 | 2 | 4 | 6 | 8 |
n(SO3)/mol | 0 | 0.8 | 1.4 | 1.8 | 1.8 |
A.ЗДгІдкЧА2 sЕФЦНОљЫйТЪІд(O2)ЃН0.4 molЁЄL1ЁЄs1
B.БЃГжЦфЫћЬѕМўВЛБфЃЌЬхЛ§бЙЫѕЕН1.0 LЃЌЦНКтГЃЪ§НЋдіДѓ
C.ЯрЭЌЮТЖШЯТЃЌЦ№ЪМЪБЯђШнЦїжаГфШы4 mol SO3ЃЌДяЦНКтЪБЃЌSO3ЕФзЊЛЏТЪДѓгк10%
D.БЃГжЮТЖШВЛБфЃЌЯђИУШнЦїжадйГфШы2 mol SO2ЁЂ1 mol O2ЃЌЗДгІДяЕНаТЦНКтЪБn(SO3)/n(O2) діДѓ
ЁОЬтФПЁПЛиД№ЯТСаЮЪЬтЃК
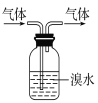
ЃЈ1ЃЉТШЛЏЬњЫЎШмвКГЪЫсадЃЌдвђЪЧЃЈгУРызгЗНГЬЪНБэЪОЃЉ_________________________ЁЃЪЕбщЪвдкСйЪБХфжЦвЛаЉТШЛЏЬњШмвКЪБЃЌГЃНЋТШЛЏЬњЙЬЬхЯШШмгкНЯХЈЕФ__________жаЃЌШЛКѓдйгУеєСѓЫЎЯЁЪЭЕНЫљашвЊЕФХЈЖШЃЌЪЧЮЊСЫвжжЦЦфЫЎНтЁЃ
ЃЈ2ЃЉ25ЁцЪБЃЌХЈЖШЮЊ0.1 mol/LЕФ6жжШмвКЃКЂйHCl ЂкCH3COOH ЂлBa(OH)2 Ђм Na2CO3 Ђн KCl ЂоNH4ClЃЌШмвКЕФpHгЩаЁЕНДѓЕФЫГађЮЊ________ЃЈЬюађКХЃЉ[вбжЊKb(NH3ЁЄH2O)=1.8ЁС10-5ЃЌKa(CH3COOH)=1.75ЁС10-5]ЁЃ
ЃЈ3ЃЉЕчРыЦНКтГЃЪ§ПЩгУРДКтСПШѕЕчНтжЪЕФЕчРыГЬЖШЁЃвбжЊШчЯТБэЪ§ОнЃЈ25ЁцЃЉЃК
ЛЏбЇЪН | HCN | CH3COOH | H2CO3 |
ЕчРыЦНКтГЃЪ§ | KЃН4.9ЁС1010 | KЃН1.8ЁС105 | K1ЃН4.4ЁС107 K2ЃН4.7ЁС1011 |
Ђй25 ЁцЪБЃЌЕШЮяжЪЕФСПХЈЖШЕФ3жжШмвКЃКaЃЎNaCNШмвК bЃЎNa2CO3ШмвК cЃЎCH3COONaШмвКЃЌЦфpHгЩДѓЕНаЁЕФЫГађЮЊ________________ЃЈЬюађКХЃЉЁЃ
Ђк25 ЁцЪБЃЌЯђNaCNШмвКжаЭЈШыЩйСПЕФCO2ЃЌЗЂЩњЗДгІЕФРызгЗНГЬЪНЮЊ_________________ЁЃ
ЃЈ4ЃЉдкЛЏбЇЗжЮіжаВЩгУK2CrO4ЮЊжИЪОМСЃЌвдAgNO3БъзМШмвКЕЮЖЈШмвКжаЕФClЃЌРћгУAg+гыCrO42ЩњГЩзЉКьЩЋГСЕэЃЌжИЪОЕНДяЕЮЖЈжеЕуЁЃЕБШмвКжаЕФClЧЁКУЭъШЋГСЕэЃЈХЈЖШЕШгк1.0ЁС105 molЁЄL1ЃЉЪБЃЌШмвКжаc(Ag+)ЮЊ__________molЁЄL1ЃЌДЫЪБШмвКжаc(CrO42)ЮЊ_________molЁЄL1ЁЃЃЈвбжЊAg2CrO4ЁЂAgClЕФKspЗжБ№ЮЊ2.0ЁС1012КЭ2.0ЁС1010ЃЉЁЃ
ЁОЬтФПЁПФГЛЏбЇбаОПадбЇЯАаЁзщЭЈЙ§ВщдФзЪСЯЃЌЩшМЦСЫШчЭМЫљЪОЕФЗНЗЈвдКЌФјЗЯДпЛЏМСЮЊдСЯРДжЦБИNiSO47H2OЁЃвбжЊФГЛЏЙЄГЇЕФКЌФјДпЛЏМСжївЊКЌгаNiЃЌЛЙКЌгаAlЃЈ31%ЃЉЁЂFeЃЈ1.3%ЃЉЕФЕЅжЪМАбѕЛЏЮяЃЌЦфЫћВЛШмдгжЪЃЈ3.3%ЃЉЁЃ

ВПЗжбєРызгвдЧтбѕЛЏЮяаЮЪНЭъШЋГСЕэЪБЕФpHШчЯТЃК
ГСЕэЮя | AlЃЈOHЃЉ3 | FeЃЈOHЃЉ3 | FeЃЈOHЃЉ2 | NiЃЈOHЃЉ2 |
pH | 5.2 | 3.2 | 9.7 | 9.2 |
ЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉВйзїaЁЂcжаашЪЙгУЕФвЧЦїГ§ЬњМмЬЈЃЈДјЬњШІЃЉЁЂОЦОЋЕЦЁЂЩеБЁЂВЃСЇАєЭтЛЙашвЊЕФжївЊвЧЦїЮЊ___ЁЃ
ЃЈ2ЃЉЁАМюНўЁБЙ§ГЬжаЗЂЩњЕФРызгЗНГЬЪНЪЧ___ЁЃ
ЃЈ3ЃЉЁАЫсНўЁБЪБЫљМгШыЕФЫсЪЧ___ЃЈЬюЛЏбЇЪНЃЉЃЎЫсНўКѓЃЌОВйзїaЗжРыГіЙЬЬхЂйКѓЃЌШмвКжаПЩФмКЌгаЕФН№ЪєРызгЪЧ___ЁЃ
ЃЈ4ЃЉВйзїbЮЊЕїНкШмвКЕФpHЃЌФуШЯЮЊpHЕФзюМбЕїПиЗЖЮЇЪЧ___ЁЃ
ЃЈ5ЃЉЁАЕїpHЮЊ2ЁЋ3ЁБЕФФПЕФЪЧ___ЁЃ
ЃЈ6ЃЉВњЦЗОЇЬхжагаЪБЛсЛьгаЩйСПТЬЗЏЃЈFeSO47H2OЃЉЃЌЦфдвђПЩФмЪЧ__ ЁЂ___ЁЃ