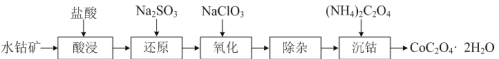
��Ŀ����
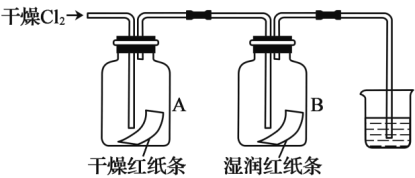
����Ŀ��ʵ�������Ҵ���Ũ���Ṳ����ȡ��ϩ�������¶ȹ��߶����� SO2���壬ʵ������������ͼ��ʾװ����֤����������Ƿ�����ϩ�� SO2 ��
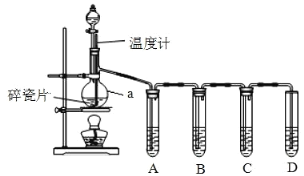
(1)���� a ��������___________�����Ƭ��������___________��
(2)����ƿ�м����Ҵ���Ũ�����˳����___________��������ϩ�Ļ�ѧ����ʽΪ___________________��
(3)A��B��C��D װ����ʢ�ŵ��Լ��ǣ�����ţ�
A__________ �� B__________ �� C__________ �� D___________��
��ѡ�Լ�����NaOH ��Һ����Ʒ����Һ�������� KMnO4 ��Һ
(4)��˵�� SO2 ������ڵ�������___________��
(5)֤��������ϩ��������___________��˵����ϩ����___________�ԡ�
���𰸡�������ƿ ��ֹ���� �ȼ��Ҵ����Ũ���� ![]() �� �� �� �� A��Ʒ����Һ��ɫ װ��C�е�Ʒ����Һ����ɫ��D�е����Ը��������Һ��ɫ ��ԭ��
�� �� �� �� A��Ʒ����Һ��ɫ װ��C�е�Ʒ����Һ����ɫ��D�е����Ը��������Һ��ɫ ��ԭ��
��������
��1��������������״�ṹ������;���н��ʵ�����Ʊ���ϩ���õ�ԭ��Ϊ�Ҵ���Ũ��������������ˮ������Ӧ�����Ǽ��ȵ�170��������Һ��ʱ�ײ������У����Լ����Ƭ��
��2��Ũ�����ܽ���ͷŴ������ȣ���ֹҺ��ɽ����ȼ��Ҵ����Ũ���ʵ���������Ҵ���Ũ����Ĵ������·�����������ˮ��ȡ��ϩ���Դ�д����ѧ����ʽ��
��3�����ֲ��������ʱ��Ӧ�����Ⱥ�˳�������������Ʒ����Һ��������ϩ�����Ը��������Һ����ϩ�Ͷ���������ʹ���Ը��������Һ��ɫ�������ȼ������������SO2�������ϩ��
��4������������ʹƷ����ɫ��
��5����ϩ��ʹ���� KMnO4 ��Һ��ɫ������Ϊ��ԭ�ԣ�����ǰ���ȳ�����������
��1��װ����a����Ϊ������ƿ��ʵ�����Ʊ���ϩ���õ�ԭ��Ϊ�Ҵ���Ũ��������������ˮ������Ӧ�����Ǽ��ȵ�170������Һ�����ʱ�ױ��У����Լ����Ƭ��ֹ���У��ʴ�Ϊ��������ƿ����ֹ���У�
��2��Ũ�����ܽ���ͷŴ������ȣ���ֹҺ��ɽ������ȼ��Ҵ����Ũ���ʵ���������Ҵ���Ũ����Ĵ������·�����������ˮ��ȡ��ϩ����ѧ����ʽΪ��![]() ���ʴ�Ϊ���ȼ��Ҵ����Ũ���
���ʴ�Ϊ���ȼ��Ҵ����Ũ��� ![]() ��
��
��3���������������Ʒ����Һ��������ϩ�����Ը��������Һ����ϩ�Ͷ���������ʹ���Ը��������Һ��ɫ�������ȼ����������Ȼ�������ϩ��ͬ�ڼ�����ϩ֮ǰ��NaOH��Һ����SO2����ͨ��Ʒ����Һ����ɫȷ��SO2�ѳ��ɾ�����������Ը��������Һ��ɫ������ϩ����װ��A��������SO2���Թ���Ʒ����Һ��ɫ��˵������SO2��װ��B�Թ�װ��NaOH��Һ��ȥSO2��װ��C�Թ�ͨ��Ʒ����Һ����ɫȷ��SO2�ѳ��ɾ���װ��Dͨ�����Ը��������Һ��ɫ������ϩ���ʴ�Ϊ���ڣ��٣��ڣ��ۣ�
��4��װ��A��������SO2���Թ���Ʒ����Һ��ɫ��˵������SO2���ʴ�Ϊ��A��Ʒ����Һ��ɫ��
��5����C����������D�������Ը��������Һ���õ�Ϊ��ϩ����ϩ����Ϊ��ԭ�ԣ�����ȷ֤������ϩ��������װ��C�е�Ʒ����Һ����ɫ��D�е����Ը��������Һ��ɫ���ʴ�Ϊ��װ��C�е�Ʒ����Һ����ɫ��D�е����Ը��������Һ��ɫ����ԭ�ԡ�

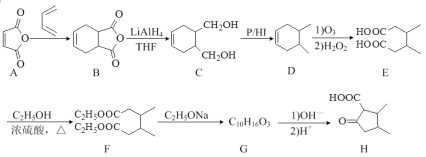
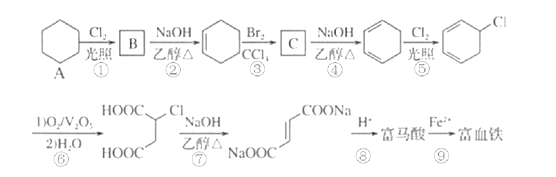
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��2020��5��1�����°桶��������������������������ʽʵʩ���������������Ӧ����ȷ����
|
|
|
|
A. �����ǡ�ʣ���� | B. ����ʹ�ú�һ���Կ��� | C. ����ҩƷ | D. һ ���Ըɵ�� |
A.AB.BC.CD.D