��Ŀ����
����Ŀ�������������������Ӧ�ù㷺��
��ij�����Ի�������Ҫ�ɷ�ΪFeS2��Ϊԭ�ϣ��������Ӵ������Ʊ����ᡣ
��֪298 K��101 kPa�����£�
2FeS2��s��=2FeS��s����S��s�� ��H1
S��s����2O2��g��=2SO2��g�� ��H2
4FeS��s����7O2��g��=2Fe2O3��s����4SO2��g�� ��H3
���ڸ�������FeS2��O2����Fe2O3��SO2�����Ȼ�ѧ����ʽ��________��
��������Ӧ��2SO2��g����O2��g��2SO3��g������H��0
��1�� ������ɱ���ܱ�������ά��ѹǿΪ1��105 Pa�ͳ�ʼn��SO2����2 mol������һ������O2��SO2ƽ��ת��������SO2����O2���ʵ���n��O2���ı仯��ϵ��ͼ��ʾ��
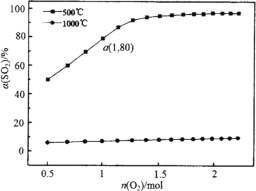
����1000��ʱ��SO2ƽ��ת��������O2���ʵ��������������ߣ�������ԭ��________��
��a��ʱSO3��ƽ���ѹp��SO3����________Pa������2λ��Ч���֣�ij��ֵ�ƽ���ѹ����ѹ��ij��ֵ����ʵ�����������
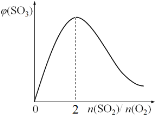
��2�������¶Ȳ��䣬��̶�������ܱ������г���һ������SO2��O2���뻭��ƽ����ϵ��SO3�������������SO3�����ʼSO2��O2�����ʵ���֮��[n��SO2��/n��O2��]�ı仯����ͼ��
��3����֪�������SO2��������ΪSO3�����ݼ����ģ��������̿�ڱ�����O2ת��Ϊ����ķ�Ӧ�����������仯��ͼ��ʾ������˵����ȷ����________��

A O2ת��Ϊ������������Ķ�����̼���������ɹ���
B �ù�����������ݣ���ܣ�E����0.73 eV
C ÿ�һ��O2����0.29 eV������
D ̿�ڿ���ΪSO2ת��ΪSO3�Ĵ���
E ����������ͬʱ��̿�ڿ���ԽС����Ӧ����Խ��
�����Ṥ��β���е�SO2�ɱ�NaOH��Һ���գ��ö��Ե缫�����õ�Na2SO3��Һ���е�⣬��ѭ������NaOH��ͬʱ�õ�H2SO4��д�������ĵ缫����ʽ________��
���𰸡�4FeS2��s����11O2��g��=2Fe2O3��s����8SO2��g�� ��H��2��H1��2��H2����H3���÷�Ӧ��H��0��1000��ʱ���÷�Ӧ�Ļ�ѧƽ�ⳣ����С��SO2ƽ��ת����С��1000��ʱ����ѹ�����£�����O2��c��SO2����c��SO3���ȱ�������c��O2������Qc�� ��K��ƽ�������ƶ���SO2ƽ��ת�������� 7.3��104��0.73��105
��K��ƽ�������ƶ���SO2ƽ��ת�������� 7.3��104��0.73��105  ADE
ADE ![]() ��H2O��2e=
��H2O��2e=![]() ��2H+
��2H+
��������
��1�����ݸ�˹���ɷ����Ȼ�ѧ����ʽ����д��
��2������ƽ�������ʽģʽ���㡣
��3�����ݷ�Ӧ���������ı仯������ܼ��Ƿ��Ȼ������ȹ��̡�
��4����������ʧȥ���ӻ��ϼ����߷����缫��Ӧ��
�� ��2FeS2��s��=2FeS��s����S��s�� ��H1����S��s����2O2��g��=2SO2��g�� ��H2����4FeS��s����7O2��g��=2Fe2O3��s����4SO2��g�� ��H3�����ݸ�˹���ɷ������١�2+�ڡ�2+�����Ȼ�ѧ����ʽΪ��4FeS2��s����11O2��g��=2Fe2O3��s����8SO2��g�� ��H��2��H1��2��H2����H3��
��(1) ���÷�Ӧ��H��0��1000��ʱ�����ݶ��������ת�������ߣ��÷�Ӧ�Ļ�ѧƽ�ⳣ����С��SO2ƽ��ת����С��1000��ʱ����ѹ�����£�����O2��c��SO2����c��SO3���ȱ�������c��O2������Qc�� ��K��ƽ�������ƶ���SO2ƽ��ת�������� ��
��K��ƽ�������ƶ���SO2ƽ��ת�������� ��
�� 2SO2(g)��O2(g)![]() 2SO3(g)
2SO3(g)
��ʼ2 1 0
�ı�1.6 0.8 1.6
ƽ��0.4 0.2 1.6
���������ƽ���ѹΪ![]() =7.3��104��0.73��105 ��
=7.3��104��0.73��105 ��
(2) �����¶Ȳ��䣬��̶�������ܱ������г���һ������SO2��O2����������������ı���Ϊ2����ʱ���������ƽ������������ͼ��Ϊ��
 ��
��
(3). A.��ͼ������O2ת��Ϊ������������Ķ�����̼���������ɹ��̣�����ȷ��
B. �ù������������(���)Ϊ0.75-0.0=0.75 eV ���ʴ���
C��ÿ�һ��O2�ͷ�0.29 eV���������ʴ���
D��̿�ڿ����ṩ���������ΪSO2ת��ΪSO3�Ĵ���������ȷ��
E������������ͬʱ��̿�ڿ���ԽС����Ӧ�Ӵ���Խ��Ӧ����Խ�죬����ȷ����ѡADE��
��.�ö��Ե缫�����õ�Na2SO3��Һ���е�⣬��ѭ������NaOH��ͬʱ�õ�H2SO4�������������������ʧȥ����������������ӣ������ĵ缫��ӦʽΪ��![]() ��H2O��2e=
��H2O��2e=![]() ��2H+��
��2H+��

 ��ĩ100�ִ��غ�������ϵ�д�
��ĩ100�ִ��غ�������ϵ�д� Сѧ�������Ծ�ϵ�д�
Сѧ�������Ծ�ϵ�д�