��Ŀ����
2������˵������ȷ���ǣ�������| A�� | ����ƿ����Ͳ�͵ζ����϶�����ʹ���¶ȣ���Ͳ������ƿ�ޡ�0���̶ȣ��ζ����С�0���̶ȣ�ʹ��ʱ�ζ���ˮϴ������ϴ��������ƿˮϴ������ϴ | |
| B�� | ���ü������ȼƲⶨ��Ӧ��ʱ����ʹ������ĭ����ȱ��µ����á���ͨ���������н���ʹ��ͼ��ַ�Ӧ��ȷ��ȡʵ��ʱ�¶ȼ�����¶ȡ�����ȡ2-3 �ε�ʵ��ƽ��ֵ�ȴ�ʩ���Դﵽ���õ�ʵ��Ч�� | |
| C�� | �����Ż�ʱ������ϸɳ������𣻵����豸����Ļ��֣���������ĭ�������� | |
| D�� | ��4mL0.1mol•L-1��K2Cr2O7��Һ�еμ�����1mol•L-1��NaOH��Һ����Һ��ɫ�ӳ�ɫ��ɻ�ɫ |
���� A�����ݱ���ʹ���¶ȵ������У��ζ��ܡ�����ƿ����Ͳ���ձ��ȣ����ݱ��С�0���̶ȵ������У��ζ��ܡ�������ƽ���¶ȼƵȣ����ݵζ��ܵ�ʹ������ȷ�����н��
B������ʹ����ͨ���������н��裬����������ɢʧ��Ӱ��ⶨ�����Ӧ��ʹ�û��β�������
C�����ķ����У���1�������������������������2�����µ���ȼ����Ż�����£���3�����߿�ȼ�
D��K2Cr2O7��Һ�д���ƽ�⣺Cr2O72-����ɫ��+H2O?2CrO42-����ɫ��+2H+��
��� �⣺A������ƿ����Ͳ�͵ζ����϶����п̶ȣ�ʹ��ʱ�����������������Ա���ʹ���¶ȣ���Ͳ��0���̶Ⱦ��ǵ���������ƿֻ�����������һ���̶ȣ��������߶��ޡ�0���̶ȣ�ʹ��ʱ�ζ���ˮϴ������ϴ��������ƿˮϴ������ϴ�����ˮ���ݣ���A��ȷ��
B���к��ȵIJⶨ�У�Ϊ�˼�������ɢʧ��Ӧ��ʹ�û��β��������裬����ʹ����ͨ����������B����
C����ĭ��������ʱ�������ĭ�к���ˮ�֣������ڵ�����·���ʱ���´��缰���������Բ�������ĭ�������𣬹�C��ȷ��
D��K2Cr2O7��Һ�д���ƽ�⣺Cr2O72-����ɫ��+H2O?2CrO42-����ɫ��+2H+���μ�����1mol/LNaOH��Һ��ƽ�������ƶ�������Һ��ɫ�ӳ�ɫ��ɻ�ɫ����D��ȷ��
��ѡB��
���� ���⿼�鳣��������ʹ�ã���Ŀ�ѶȲ���ע�ⳣ����ѧ������ʹ�÷�����ע�����

 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д���a=b+4����a+b=8����a+b=30����a=b+8��
| A�� | 1�� | B�� | 2�� | C�� | 3�� | D�� | 4�� |
| Q | R | ||
| T | W | ||
| A�� | ��̬�⻯���ȶ��ԣ�R��Q | |
| B�� | Ԫ��T�����ӽṹʾ��ͼΪ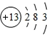 | |
| C�� | ��Wͬ�����ijԪ���γɵ�18���ӵ��⻯������м��м��Լ����зǼ��Լ� | |
| D�� | W���γɶ��ֺ����� |
| �� | �� | �� | �� | �� | �� | �� | �� | |
| ԭ�Ӱ뾶��10-10m�� | 0.74 | 1.60 | 1.52 | 1.10 | 0.99 | 1.86 | 0.75 | 1.43 |
| �����ͻ��ϼ� | +2 | +1 | +5 | +7 | +1 | +5 | +3 | |
| -2 | -3 | -1 | -3 |
��1���۵�Ԫ�ط�����Li���������������Ӧˮ������Al��OH��3��
����Ԫ�����ڱ��е�λ���ǣ����ڡ��壩�ڶ����ڢ�A�壮
��2��������ǿ�Ļ�����ĵ���ʽ�ǣ�
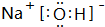 �������ӻ��������ӡ����ۡ�����
�������ӻ��������ӡ����ۡ�������3���ȽϢܺ͢ߵ��⻯����ȶ��ԣ��û�ѧʽ��ʾ��NH3��PH3��
��4��д���ߵ�����������Ӧˮ����������⻯�ﷴӦ�Ļ�ѧ����ʽ��NH3+HNO3=NH4NO3��
��5��д��������������Ӧˮ������ݵ��⻯��ˮ��Һ��Ӧ�����ӷ���ʽ��Mg��OH��2+2H+=Mg2++2H2O��
| A�� | ������Һ����ǿ�����ԣ���������ˮ�� | |
| B�� | �������費���κ��ᷴӦ������ʯӢ������������ | |
| C�� | ͭ�Ľ�����Ա����������ں��������װ����ͭ���Լ����丯ʴ | |
| D�� | �����£����ܱ�Ũ����ۻ����������Ʋ۳�����Ũ���� |
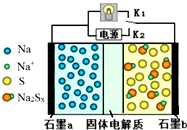 ������������һ�����Ϳɳ���أ��乤��ԭ����ͼ��ʾ��ͼ�й���������Na+���壮����������ȷ���ǣ�������
������������һ�����Ϳɳ���أ��乤��ԭ����ͼ��ʾ��ͼ�й���������Na+���壮����������ȷ���ǣ�������| A�� | �ŵ�ʱ��ʯī�缫aΪ���� | |
| B�� | �ŵ�ʱ��Na+��ʯīb��ʯīa����Ǩ�� | |
| C�� | ���ʱ��b����ӦΪNa2Sx-2e-=xS+2Na+ | |
| D�� | �ɽ�װ���еĹ������ʸij�NaCl��Һ |


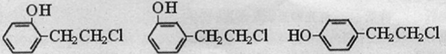 ��
�� ��
�� ����д�����漰�ķ�Ӧ����ע����Ӧ��������
����д�����漰�ķ�Ӧ����ע����Ӧ�������� ��
��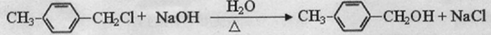 ��
��