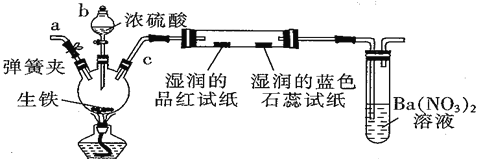
��Ŀ����
����Ŀ�����Ȼ���(TiCl4)����ȡ���캽�չ�ҵ���ϡ����ѺϽ����Ҫԭ�ϡ���������(��Ҫ�ɷ���FeTiO3)�Ʊ�TiCl4��ͬʱ��ø���Ʒ�Ĺ�ҵ�����������£�
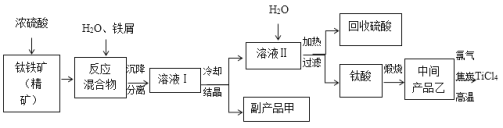
��1����֪�����ӦFeTiO3+2H2SO4===FeSO4+TiOSO4+2H2O����FeTiO3����Ԫ�صĻ��ϼ�Ϊ___________��
��2���������������м�����м��Ŀ����ʹFe3+��ԭΪFe2+���ҷ�Ӧ�õ�����Һ�к���Ԫ�ص�����ֻ��TiO2+���ù��̷�������Ҫ��Ӧ�У�
��2Fe3++Fe===3Fe2+ �� ��2TiO2++Fe+4H+===2Ti3++Fe2++2H2O����______________________________________��
��3������TiOSO4��Һ���Ʊ�TiO2��nH2O���壬�䷴Ӧ�Ļ�ѧ����ʽΪ__________________��
��4����TiO2��nH2O�����Ƶù���TiO2��nH2O����������ϴ��ȥ���е�Fe(OH)3���ʣ������Ƶ��Ѱۡ���֪25��ʱ��Ksp[Fe(OH)3]��2.79��10��39�����¶��·�ӦFe(OH)3+3H+![]() Fe3++3H2O��ƽ�ⳣ��K��___________________��
Fe3++3H2O��ƽ�ⳣ��K��___________________��
��5����ѭ�����õ�������_________������Ʒ���ᾧˮ���仯ѧʽ��_______________��
��6�������±���Ϣ��Ҫ���ƺ�����SiCl4���ʵ�TiCl4���ɲ���___________������
TiCl4 | SiCl4 | |
�۵�/�� | ��25.0 | ��68.8 |
�е�/�� | 136.4 | 57.6 |
���𰸡�+2 Ti3����Fe3����H2O=TiO2����Fe2����2H�� TiOSO4+(n+1)H2O![]() TiO2��nH2O(����)+H2SO4 2.79��103 H2SO4 FeSO4��7H2O ����
TiO2��nH2O(����)+H2SO4 2.79��103 H2SO4 FeSO4��7H2O ����
��������
�������м���ǿ�ᣬǿ���Խ���Һ�к���TiO2+��Fe2+��Fe3+�ȣ��ټ�����м��ַ�Ӧ���ù����������·�Ӧ������2Fe3++Fe=3Fe2+��2TiO2++Fe+4H+=2Ti3++Fe2++2H2O��Ti3����Fe3����H2O=TiO2����Fe2����2H������ҺI�������õ�����Ʒ�̷�FeSO4��7H2O�ͺ���TiO2+����ҺII������ҺII�����õ�TiO2nH2O�����պ�TiO2����������̿��ϼ��ȵõ�TiCl4��������ӦTiO2+2C+2Cl2![]() TiCl4+2CO���������������ݴ˷������
TiCl4+2CO���������������ݴ˷������
(1) FeTiO3���ѵĻ��ϼ�Ϊ+4�ۣ���Ϊ-2�ۣ����ݻ������и�Ԫ�ػ��ϼ۵Ĵ�����Ϊ0������Ԫ�صĻ��ϼ�Ϊ+2�ۣ�
(2)��Ӧ�õ�����Һ�к���Ԫ�ص�����ֻ��TiO2�����ù���Ti3���������Fe3����������TiO2��������������ԭ��Ӧ����ƽ�÷�ӦΪTi3����Fe3����H2O=TiO2����Fe2����2H����
(3)����TiOSO4��Һˮ����Ʊ�TiO2��nH2O ���壬�䷴Ӧ�Ļ�ѧ����ʽΪTiOSO4+(n+1)H2O![]() TiO2��nH2O(����)+H2SO4��
TiO2��nH2O(����)+H2SO4��
(4)��Ksp[Fe(OH)3]��2.79��10��39�ɵ�c(Fe3��)��c3(OH��)��2.79��10��39����ӦFe(OH)3��3H��![]() Fe3����3H2O��ƽ�ⳣ��K��
Fe3����3H2O��ƽ�ⳣ��K��![]() =
=![]() =
=![]() =
=![]() =2.79��103��
=2.79��103��
(5)��Һ�����ȹ��˺�������ᣬ���ܽ�������ʱ��Ũ���ᣬ�ʿ�ѭ�����õ�������H2SO4����Һ����ȴ�ᾧ��������������������Ʒ���ᾧˮ���仯ѧʽ��FeSO4��7H2O��
(6)���ݱ�����Ϣ��TiCl4��SiCl4�ķе����ܴ����Բ�������ķ������з�����

����Ŀ��������ͼ��ʾװ�òⶨ�к��ȵ�ʵ�鲽�����£�
������Ͳ��ȡ50 mL 0.50 mol��L��1���ᵹ��С�ձ��У���������¶ȣ�
������һ��Ͳ��ȡ50 mL 0.55 mol��L��1 NaOH��Һ������ͬһ�¶ȼƲ�����¶ȣ�
�۽�NaOH��Һ����С�ձ��У��跨ʹ֮��Ͼ��ȣ���û��Һ����¶ȡ��ش��������⣺

��1����ͼ��������δ������������______��__________��
��2��Ϊʲô����NaOH��ҺҪ�Թ���_________________��
��3������NaOH��Һ����ȷ������_______________________��
��4���ֽ�һ������ϡ����������Һ��ϡ����������Һ��ϡ��ˮ�ֱ��1 L 1 mol��L��1��ϡ����ǡ����ȫ��Ӧ���䷴Ӧ�ȷֱ�Ϊ��H1����H2����H3������H1����H2����H3�Ĵ�С��ϵΪ______��
��5���������������������Һ���ܶȶ���1 g��cm��3����֪�кͷ�Ӧ��������Һ�ı�����c��4.18 J��g��1������1��Ϊ�˼����к��ȣ�ijѧ��ʵ���¼�������£�
ʵ�� ��� | ��ʼ�¶�t1/ �� | ��ֹ�¶�t2/ �� | |
���� | ����������Һ | �����Һ | |
1 | 20.0 | 20.1 | 23.2 |
2 | 20.2 | 20.4 | 23.4 |
3 | 20.5 | 20.6 | 23.6 |
���ݸ�ѧ����ʵ�����ݼ��㣬��ʵ���õ��к�����H��______(�������һλС��)��
��6��____(������������������)��Ba(OH)2��Һ�������������������Һ�����ᣬ������______��
����Ŀ��ij̽��С����KMnO4������Һ��H2C2O4��Һ��Ӧ��������Һ��ɫ��ʧ�ķ������о�Ӱ�췴Ӧ���ʵ����أ�
��1���÷�Ӧ�����ӷ���ʽΪ __________________________________ ��
ʵ��������������������KMnO4������Һ��Ũ�ȿ�ѡ��0.01molL-1��0.001molL-1��ÿ��ʵ��KMnO4������Һ��������Ϊ4mL��H2C2O4��Һ��0.1molL-1����������Ϊ2mL��������������ͬ������£�ijͬѧ�ı�KMnO4������Һ��Ũ�ȣ��������ʵ�����ݣ��ӻ�����ȿ�ʼ��ʱ����
KMnO4������Һ��Ũ��/molL-1 | ��Һ��ɫ����ʱ��t/min | ||
��1�� | ��2�� | ��3�� | |
0.01 | 14 | 13 | 11 |
0.001 | 6 | 7 | 7 |
��2��������0.001molL-1KMnO4������Һ����ʵ��ʱKMnO4��ƽ����Ӧ����___________________________ �����Ի��ǰ����Һ������仯����
��3�������������㣬ֱ�ӿ����е���ɫʱ�䳤�����ж�Ũ�ȴ�С�뷴Ӧ���ʵĹ�ϵ�Ƿ���У� _____________________ ������������������������