��Ŀ����
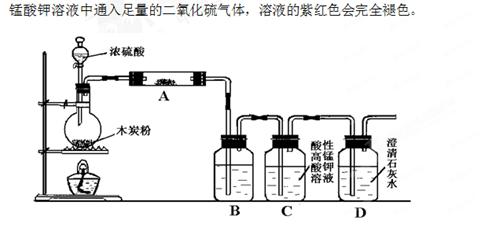
��16�֣���ԭ������������ƣ�Na2S2O3���ڹ�ҵ������ҽҩ����ҵ�б��㷺Ӧ�ã���ҵ�ձ�ʹ��Na2SO3����ǣ�S������õ���װ����ͼ1��
��֪��Na2S2O3��������Һ�в����ȶ����ڡ�
��1������1����K1���ر�K2����Բ����ƿ�м��������ײ����ȣ����Լ���Ϊ��
��
��2������2��ʼ�ձ���C����Һ�ʼ��ԣ���Ӧһ��ʱ�����۵������٣���K2���ر�K1��ֹͣ���ȡ�
��C����Һ�뱣�ֳʼ��Ե�ԭ���������ԣ��� ���� ������������ ��
���������ӷ���ʽ��ʾ��
��װ��B��D�������� ��
����3����C�����û��������ᴿ��ò�Ʒ��
��3�����÷�Ӧ2Na2S+Na2CO3+4SO2=3Na2S2O3+CO2Ҳ���Ʊ�Na2S2O3������������ͼ2���������������Ӹ��������ӿ�˳��Ϊ�� ��g��h�� �� �� �� ��d��
��4��װ����ʢװ���Լ��ǣ�_____________________________��
��5��Na2S2O3��ԭ�Խ�ǿ����ҵ�ϳ�������ȥ��Һ�в�����Cl2���÷�Ӧ�����ӷ���
ʽΪ�� ����
��6������Ƽ�ʵ�鷽����֤������������Cl2����ԭ����Cl����____________
��
��16 �֣�ÿ��2�֣�
��1��Ũ����
��2�� ��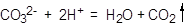 ��
��
�� ����SO2����ֹ��Ⱦ
��3�� a ��g��h�� b �� c �� e �� f ��d��
��4�� Na2CO3��Na2S�Ļ����Һ
��5��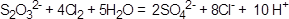
��6�� ȡ������Ӧ�����Һ���Թ��У�����Ba(NO3)2��Һ�����ڲ�����������ȡ�ϲ���Һ������˺�ȡ��Һ�����μ�AgNO3��Һ����������ɫ��������˵��Cl2����ԭΪ��Cl����
���������������1��Na2SO3����ǣ�S��������Ƶ���������ƣ�װ��C���Լ�ΪNa2CO3��Һ����ۣ������Լ�����ͭƬ��Ӧ����SO2�����Լ���ΪŨ���ᡣ
��2����Na2S2O3��������Һ�в����ȶ����ڣ�����C����Һ�������ԣ�������Ӧ��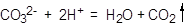 ��
��
��β���е�SO2�Ǵ�����Ⱦ�����װ��B��D�������ǣ�����SO2����ֹ��Ⱦ��
��3��a��g��h����Ӧ����ʱͨ��a����β�����գ�Ȼ���b��c������һ��������װ�ã���e�������У�SO2��װ�����Լ�������Ӧ��ȡ��������ƣ�Ȼ��f��d������β���������ʽӿ�˳��Ϊ��a��g��h��b��c����e��f��d��
��4����Ϊ��ȡ��������Ƶ�װ�ã�ͨ������ͨ��SO2����װ����ʢװ���Լ���������Ӧ�Na2CO3��Na2S�Ļ����Һ��
��5��Na2S2O3��ԭ�Խ�ǿ����Cl2����������ԭ��Ӧ������SO42?��Cl?��H+�����ݻ��ϼ���������ƽ�ɵ����ӷ���ʽ��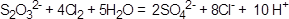 ��
��
��6����Ӧ�����Һ����SO42?�������Cl?�ļ��飬����Ӧ����������Ba(NO3)2��Һ��ȥSO42?��Ȼ���ٽ���Cl?�ļ��飬��ʵ�鷽��Ϊ��ȡ������Ӧ�����Һ���Թ��У�����Ba(NO3)2��Һ�����ڲ�����������ȡ�ϲ���Һ������˺�ȡ��Һ�����μ�AgNO3��Һ����������ɫ��������˵��Cl2����ԭΪ��Cl����
���㣺���⿼��ʵ�鷽���ķ�������ơ����ӷ���ʽ����д�����ӵļ��顣

 �Ķ��쳵ϵ�д�
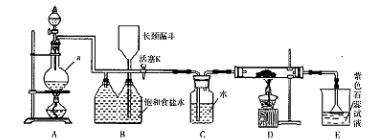
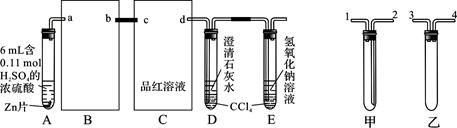
�Ķ��쳵ϵ�д�ij��ѧ��ȤС����ʵ����̽�������й����ʣ��������ͼ��ʾʵ�飬A�������巢��װ�ã�A�����õ�ʵ��ҩƷ������������ѡȡ��
��̼���� ��̼������ ��̼����� ���Ȼ�� ����ʯ�� ����������
����ͼ���Ӻø��������ֽ�C���IJ�˿�����������ȣ��ٽ�A������������ͨ��Bװ��Ƭ�̺�ȥC���ƾ��ƣ�����ʵ����������£���˿�������ֺ���״̬��D�е�ͭƬ�����ܽ⣬�ش��������⣺
��1����A����ȡ����ʱֻ����һ��ҩƷ�����ҩƷ�� ����������ţ�
��2��Bװ�õ�����Ϊ
��3��D��ͭƬ������Ӧ�����ӷ���ʽΪ ��Ϊ��ʹCuƬ�ܽ�����ʼӿ죬����D������Һ�м����������������е� �������и�����ţ�
| A��Na2CO3 | B��AgNO3 | C��H2SO4 | D��FeSO4 |
��5��������Ϊ������Ƶ�����ʵ��װ�û��������ԵIJ��㣬���䲻��֮���Լ�Ӧ��θĽ���̸̸�������� ��

 2NO
2NO CaCl2+2NH3��+2H2O
CaCl2+2NH3��+2H2O
 Na2SO4+MnSO4+Br2��+2H2O
Na2SO4+MnSO4+Br2��+2H2O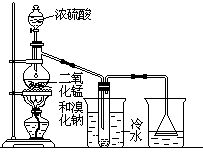

 R-CH��OH��SO3Na
R-CH��OH��SO3Na