��Ŀ����
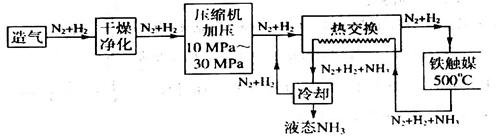
�Ӵ������������Ȱ�SO2��������SO3��Ȼ����Ũ�������յõ���SO3��ȡ��Ʒ��ij������������ʱ������Ӵ��ҵ�ԭ�����ɷ�ΪSO27%��O2 11%��N2 82%�������������
��1�������״����10 m3ԭ�����е�SO2���ʵ���______________mol��
��2�������״����1 0m3ԭ���������� ǧ�ˡ�
��3����SO2��ת����Ϊ99��2%������Ӵ��ҵ�����������SO3��������� ��
��4�����Ӵ��ҵ����������к�6��72���������������SO3���ѳ��������ͽ�����������
98��3%���������գ��ɵõ�������H2SO4����H2SO4��SO3�Ļ������к���������Ϊ20����SO3������������1000 m3�������壨������Ϊ��״��������Ҫ��98��3%����������� ǧ�ˡ�
��1��31��25��2�֣�
��2��13��82Kg��4�֣�
��3��7��19%��4�֣�
��4��665Kg��6�֣�
���������������1��ԭ������SO2���������Ϊ7%����10m3ԭ�����е�SO2�����Ϊ10m3��7%=0��7m3=700L���ʱ�״���¶�����������ʵ���Ϊ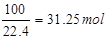 �ʴ�Ϊ��31��25��
�ʴ�Ϊ��31��25��
��2��ԭ������O2���������Ϊ11%����10m3ԭ�����е�O2�����Ϊ10m3��11%=1��1m3=1100L���ʱ�״�������������ʵ���Ϊ ԭ������N2���������Ϊ82%����10m3ԭ�����е�N2�����Ϊ10m3��82%=8��2m3=8200L���ʱ�״���µ��������ʵ���Ϊ
ԭ������N2���������Ϊ82%����10m3ԭ�����е�N2�����Ϊ10m3��82%=8��2m3=8200L���ʱ�״���µ��������ʵ���Ϊ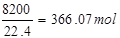 �ʱ�״����10m3ԭ����������Ϊ��31��25mol��64g/mol+49��11mol��32g/mol+366��07mol��28g/mol=13821��48��13��82kg���𣺱�״����10m3ԭ����������Ϊ13��82kg��
�ʱ�״����10m3ԭ����������Ϊ��31��25mol��64g/mol+49��11mol��32g/mol+366��07mol��28g/mol=13821��48��13��82kg���𣺱�״����10m3ԭ����������Ϊ13��82kg��
��3��SO2��ת����Ϊ99��2%����μӷ�Ӧ�Ķ�����������Ϊ10m3��7%��99��2%=0��6944m3����
2SO2+O2=2SO3 ������١�V
2 2 1
0��6944m3 0��6944m3 0��3472m3
�ʷ�Ӧ����������Ϊ10m3-0��3472m3=9��6528m3��
�ʽӴ��ҵ�����������SO3�����Ϊ0��6944m3���������Ϊ
�𣺽Ӵ��ҵ�����������SO3���������Ϊ7��19%��
��4��������ҪŨ���������Ϊmg����Ũ���������������Ϊmg��98g%=0��98mg�����ʵ���Ϊ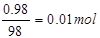 Ũ������ˮ������Ϊmg-0��98mg=0��02mg�����ʵ���Ϊ
Ũ������ˮ������Ϊmg-0��98mg=0��02mg�����ʵ���Ϊ ������������������ɵ���������ʵ���Ϊ
������������������ɵ���������ʵ���Ϊ ������������������ʵ���Ϊ0��01m mol+
������������������ʵ���Ϊ0��01m mol+ 1000m3����������������������Ϊ1000m3��6��72%=67��2m3=67200L��SO3�����ʵ���Ϊ
1000m3����������������������Ϊ1000m3��6��72%=67��2m3=67200L��SO3�����ʵ���Ϊ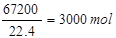 ��ˮ���պ�ʣ���������������ʵ���Ϊ3000mol-
��ˮ���պ�ʣ���������������ʵ���Ϊ3000mol-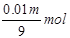
�� ,���m=664615g=664��6kg��
,���m=664615g=664��6kg��
������1000m3������������Ҫ��98%�����������Ϊ664��6kg��
���㣺���⿼��������йؼ��㡢���ݷ���ʽ���йؼ��㣬���̸��ӡ��������ܴ�Ϊ�״���Ŀ���ѶȽϴ�

���⣨H2S����һ�־��г�������ζ����ɫ���壬�о綾�������ڶ������������Լ���Ȼ���С�������ĺܶ�����������Ҳ������Ҫ���á�
| ���ϣ��� H2S������ˮ��Լ1��2������ˮ��ҺΪ��Ԫ���ᡣ �� H2S��������������ӷ�Ӧ���ɳ����� �� H2S�ڿ�����ȼ�գ�����ʵ���ɫ�� |
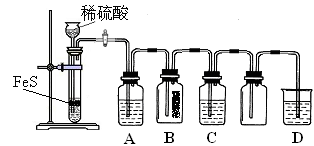
�ش��������⣺
�� A���к�ɫ������CuS��������A�з�����Ӧ�Ļ�ѧ����ʽΪ_________________��
�� B�������_________��
�� C��ֻ��dz��ɫ��������������Һ��dz��ɫ����C�з�����Ӧ�����ӷ���ʽΪ_____��
�� D��ʢ�ŵ��Լ�������____________�����ţ���
a. ˮ b. ���� c. NaCl��Һ d. NaOH��Һ
��2��Ϊ��һ��̽��-2����Ļ�������+4����Ļ����ﷴӦ������С��ͬѧ�����������ʵ�顣
| | ʵ����� | ʵ������ |
| ʵ��1 | ����Ũ�ȵ�Na2S��Na2SO3��Һ�������2��1��� | ���������� |
| ʵ��2 | ��H2Sͨ��Na2SO3��Һ�� | δ�����Գ������ټ�������ϡ���ᣬ������������dz��ɫ���� |
| ʵ��3 | ��SO2ͨ��Na2S��Һ�� | ��dz��ɫ�������� |
��֪������ƽ�ⳣ����H2S Ka1 =1.3��10-7��Ka2 = 7.1��10-15
H2SO3 Ka1 =1.7��10-2��Ka2 = 5.6��10-8
�� ��������ʵ�飬���Եó����ۣ���_________�����£�+4����Ļ������������-2����Ļ����
�ڽ�SO2����ͨ��H2Sˮ��Һ��ֱ�����������б�ʾ��ҺpH��SO2��������仯��ϵʾ��ͼ��ȷ����______������ţ���
 |  |  |  |
| A | B | C | D |
��3�������أ�������H2S����Ag�����û���Ӧ����H2���ֽ�H2S����ͨ��װ�����۵IJ����ܣ�����Ƽ�ʵ�飬ͨ�����鷴Ӧ����֤��H2S��Ag�������û���Ӧ_______��
��15�֣�
ij�о�С�����о�̼��Ũ����ķ�Ӧ����ʵ��������¡�
| ���� | ���� |
 a���ø���ྻ���ձ�ȡԼ10 mLŨ���ᣬ���ȡ� a���ø���ྻ���ձ�ȡԼ10 mLŨ���ᣬ���ȡ� | |
| b����С���պ��ľ̿Ѹ�������ȵ�Ũ�����С� | ���ȵ�ľ̿���ȵ�Ũ����Ӵ��������ҷ�Ӧ��ͬʱ�д�������ɫ���������Һ����ľ̿Ѹ��ȼ�գ����������� |
�� ̼��Ũ����ķ�Ӧ��˵��Ũ������� �ԡ�
�� ��Ӧ������������ʹ����Ũ�������ȷֽ⣬��������ɫ���塢һ����ɫ��ζ�ĵ�������X��ˮ������X�Ļ�ѧʽ�� ��
��2��ʵ��������Һ����ľ̿Ѹ��ȼ�գ�����������ͬѧ����Ϊ������ľ̿������X��Ӧ����������ͬѧ�Ҳ²�NO2���ܾ�����ȼ�ԣ�ľ̿����NO2��ȼ�ա��������������ʵ�顣
��.��ȡNO2���塣
�� �����߿��ڻ�����ͭ��Ũ������ȡ���ռ�NO2��װ�ü�ͼ(�г�������)��

�� NaOH��Һ�����������ն����NO2���÷�Ӧ�����������ʵ�����ȵ����Σ�д����Ӧ�Ļ�ѧ����ʽ ��
��.̽��ʵ�顣
ʵ��������ڿ�������ȼľ̿��ʹ��ȼ�ղ����л��棬���������ľ̿����ʢ��NO2����ļ���ƿ�С�
ʵ������ľ̿��NO2�����г���ȼ�գ�����Ѹ�ٱ���������ƿ��������ɫ��dzֱ����ɫ��������������ʹ����ʯ��ˮ����ǣ�������������ɫ��
�� ����ʵ������д��̼��NO2���巴Ӧ�Ļ�ѧ����ʽ ��
�� �Է����Ƿ���Ҫ���Ӵ������ľ̿�봿����X���巴Ӧ��ʵ�� ��
�� ͨ��ʵ��̽��������Ϊ�ס���ͬѧ��Ԥ���Ƿ��������������� ��
�����ǻ�ѧ��ҵ��Ӧ�÷dz��㷺�����ʡ���������������������Ҫ��;��
�������Ƽ���ķ���Ϊ�����й�������ҵ��������Ҫ���ס��Ƽ�ĵ�һ����Ӧ���Ͱ�����ˮ��ͨ�������̼���÷�Ӧ�ɱ�ʾΪ��NaCl + CO2 + NH3 + H2O �� NaHCO3��+ NH4Cl
����45��ʱ��ȡ117gʳ�����Ƴɱ�����Һ��������ͨ��������������������ͨ�������̼��ʹ��Ӧ������ȫ���Լ��㲢�ش��������⣨������ȡ��λ��Ч���֣����й����ʵ��ܽ�������������λ��g/100gˮ����
| | NaCl | NaHCO3 | NH4Cl |
| 10�� | 35.8 | 8.15 | 33.0 |
| 45�� | 37.0 | 14.0 | 50.0 |
��1��117gʳ�������Ͽ�����ȡ���� g��
��2��45�淴Ӧ��Ϻ��о�����������Һ��ʣ��ˮ g��������������� g��
��3�����˳�ȥ�����ľ�����ٽ�����10�棬���о������������������������������ ��
��ҵ������Ҳ�ǰ�����Ҫ��;֮һ����Ӧ���£�
4NH3+5O2��4NO+6H2O 2NO+O2��2NO2 3NO2+H2O��2HNO3+NO
��a mol��NH3��b mol��O2��Ϻ���һ�ܱ���������Pt������������700�棬��ַ�Ӧ����ȴ�����¡�
 ��4��������b�Ma��ȡֵ��Χ����֮��Ӧ����Һ�����ʼ������ʵ���������������±��У�
��4��������b�Ma��ȡֵ��Χ����֮��Ӧ����Һ�����ʼ������ʵ���������������±��У�| b�Ma��ȡֵ��Χ | ���� | �������ʵ��� |
| | | |
 | ���� | ���� |
| | | |
| | | |