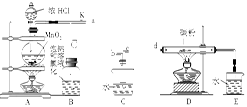��Ŀ����
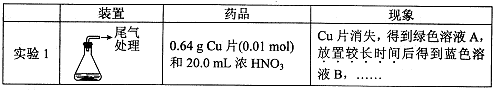
ij��ѧ��ȤС����ʵ����̽�������й����ʣ��������ͼ��ʾʵ�飬A�������巢��װ�ã�A�����õ�ʵ��ҩƷ������������ѡȡ��
��̼���� ��̼������ ��̼����� ���Ȼ�� ����ʯ�� ����������
����ͼ���Ӻø��������ֽ�C���IJ�˿�����������ȣ��ٽ�A������������ͨ��Bװ��Ƭ�̺�ȥC���ƾ��ƣ�����ʵ����������£���˿�������ֺ���״̬��D�е�ͭƬ�����ܽ⣬�ش��������⣺
��1����A����ȡ����ʱֻ����һ��ҩƷ�����ҩƷ�� ����������ţ�
��2��Bװ�õ�����Ϊ
��3��D��ͭƬ������Ӧ�����ӷ���ʽΪ ��Ϊ��ʹCuƬ�ܽ�����ʼӿ죬����D������Һ�м����������������е� �������и�����ţ�
| A��Na2CO3 | B��AgNO3 | C��H2SO4 | D��FeSO4 |
��5��������Ϊ������Ƶ�����ʵ��װ�û��������ԵIJ��㣬���䲻��֮���Լ�Ӧ��θĽ���̸̸�������� ��
��1���ۣ�2�����ն�����̼��ˮ��������������
��3��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O B C
��4��ʹ�к��������ո���ȫ
��5��Dװ�õ�Һ�������벣����C�У�ʹ���������ѣ���Cװ����Dװ��֮������һ������װ�á�
��������������������װ��ͼ��C��D�з�Ӧ����֪����ʵ��Ϊ̽�����Ĵ�������������ͭ�ķ�Ӧ��װ��A������Ϊ���ɰ�����װ��B������Ϊ��Ӧ�ṩ������װ��E��FΪβ������װ�á���1��������������֪��A�����ɵ������к��а����������Ʒ�Ӧ�������������������̼��ˮ������A����ȡ����ʱֻ����һ��ҩƷ������ѡ���֪һ���Լ����ɰ����Ͷ�����̼���Լ�ѡ��̼����泥���Ϊ���ۣ���2��Bװ�������ù�����������̼����立ֽ����ɵĶ�����̼��ˮ����������������Ϊ�����ն�����̼��ˮ������������������3��D�з�ӦΪͭƬ����ϡ������������ͭ��һ��������ˮ�����ӷ���ʽΪ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O��ʹCuƬ�ܽ�����ʼӿ죬��������ԭ���ԭ������������Ũ�ȵķ�����A��Na2CO3 �������ᣬ����Ũ�ȼ�С����Ӧ���ʼ���������B��AgNO3 ��ͭ��Ӧ��������ͭ������ϡ������Һ������ԭ��ؼӿ췴Ӧ���ʣ���ȷ��C��H2SO4 ������������Ũ�ȼӿ�ͭ��ϡ����ķ�Ӧ���ʣ���ȷ��D��FeSO4����������ܼӿ�ͭ�ķ�Ӧ���ʣ�����ѡBC����4��ͼE�г���ͨ��������������ʹ���ɵ�һ����������ȫ��ת��Ϊ���������ȫ���գ���Ϊ��ʹ�к��������ո���ȫ����5��Dװ���е�Һ������������Cװ�ã���Ҫ��CD֮���һ����������װ�ã���Ϊ��Dװ�õ�Һ�������粣����C�У�ʹ���������ѣ���C��Dװ��֮������һ����ֹ������װ�á�
���㣺���鰱�����Ʊ������ʡ�ʵ�鷽���ķ��������ۡ�

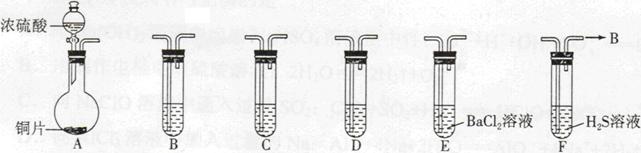
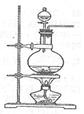
��ͼ���о�ͭ��Ũ����ķ�Ӧװ�ã�

��1��A�Թ��з�����Ӧ�Ļ�ѧ����ʽΪ ��
��2����Ӧһ��ʱ��ɹ۲쵽B�Թ��е�����Ϊ ��
��3��C�Թܿڽ���NaOH��Һ������������ ��
��4���罫B�Թܻ���D�Թܣ�����ֱ����������BaCl2��Һ��ͨ����һ�����壬������ɫ����������������� �� ����Ҫ����һ�ֻ������һ�ֵ��ʵĻ�ѧʽ��������Ҫ���ɼ�װ������װ�á���
��5��ʵ�������֤��A�Թ��з�Ӧ���ò����Ƿ���ͭ���ӵIJ��������� ��
��6����ͭ��Ũ���ᷴӦ�Ĺ����У������к�ɫ���ʳ��֣�������������������ϡ�
����
| ����1 |  ����ͭ��Ũ���ᷴӦ������ɫ���ʵ�������� |
| ����2 | X���߾������������ͭ��Ũ���ᷴӦ���ɵĺ�ɫ����ΪCu2S��CuS��Cu7S4�е�һ�ֻ��֡� |
�������Ͽɵó�����ȷ������ ��
a��ͭ��Ũ���ᷴӦʱ���漰�ķ�Ӧ���ܲ�ֹһ��
b������Ũ��ѡ���ʵ����ɱ����������г��ֺ�ɫ����
c���÷�Ӧ����������֮һ������Ũ�ȡ�15 mol��L
d������Ũ��Խ��ɫ����Խ����֡�Խ����ʧ
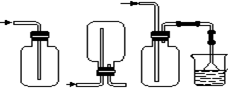
ij������ȤС��Ϊ̽��ͭ��Ũ���ᷴӦ���������ͼ��ʾװ�ý���ʵ�顣��֪����SO2�����ڱ���������������Һ����SO2�������Ը��������Һ����������ԭ��Ӧʹ֮��ɫ����ѧ����ʽΪ5SO2+2KMnO4+2H2O=K2SO4+2MnSO4+2H2SO4����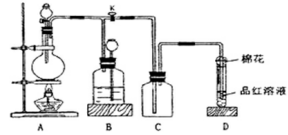
�ش��������⣨ע��EΪֹˮ�У�FΪ��������
��1�����Aװ�õ������Եķ��� ��
��2��װ��A�з�Ӧ�Ļ�ѧ����ʽΪ ��
��3��װ��D���Թܿڷ��õ���Ӧպ��NaOH��Һ��
�������� ��
��4��װ��B����������������á���D�������Ե�����ر�����F����ȥ�ƾ��ƣ��������ȵ����ã�A�����������������ʱB�е������� ��B��Ӧ���õ�Һ���ǣ�����ĸ�� ��
| A��ˮ | B������NaHSO3��Һ | C������KMnO4��Һ | D��NaOH��Һ |
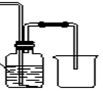
ij����С��������CuO��NH3��Ӧ,�о�NH3��ij�����ʲ��ⶨ�����,���������ʵ��װ�ã��г�װ��δ����������ʵ�顣��ش���������: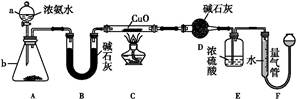
��1������a������Ϊ��������;����b�п�ѡ����Լ�Ϊ����������
��2��ʵ������,����װ��A,������ȡ����ɫ�������������� ������ĸ����
| A��Cl2 | B��O2 | C��CO2 | D��NO2 |
��4��Eװ����Ũ������������� ��
��5����ȡ�������ǰ,Ӧ��װ��F���еIJ���: ����
��6��ʵ�����,����ø����D����m g,װ��F�����������Ϊn L��������ɱ�״����,�����е������ԭ�Ӹ�����Ϊ�����������ú�m��n��ĸ�Ĵ���ʽ��ʾ����


 Cu��NO2��42-����ɫ��
Cu��NO2��42-����ɫ��