��Ŀ����
����Ŀ��I.2017��5��5�գ��й��ܰ��չ��ʱ����Ƶ�ӵ������֪ʶ��Ȩ�Ĵ��Ϳͻ�C-919���Ϻ��ֶ������ɣ���ѧ����ʵ�������о������ô��������ɻ�β���е�NO��COת���CO2��N2�ķ�ӦΪ2NO(g)+2CO(g)![]() N2(g)+2CO2(g)��H<0��
N2(g)+2CO2(g)��H<0��
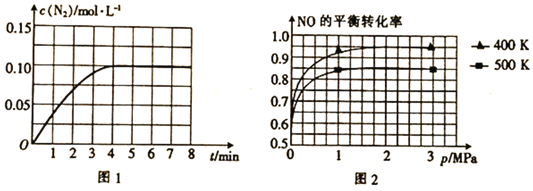
(1)����1mol NO��2mol COͨ��2L�ĺ����ܱ������У���һ�������·���������Ӧ����Ӧ�����ɵ�N2�����ʵ���Ũ����ʱ��ı仯�����ͼ1��ʾ����NO�ӷ�Ӧ��ʼ��ƽ��ʱ��ƽ����Ӧ����v(NO)=__________��4minĩCO��Ũ��Ϊ__________mol��L-1��
(2)��֪������Ӧ��NO��ƽ��ת������ѹǿ���¶ȵĹ�ϵ��ͼ2��ʾ����ҵ�ϴ�װ���бȽ��ʺϵ��¶Ⱥ�ѹǿ��__________
II.ȡ50 mL0.50mol��L-1NaOH��Һ��50 mL��0.50mol/L������Һ�����к��Ȳⶨʵ�飬ʵ���������±���
(1)����д���еĿհף�
�¶� ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | �¶Ȳ�ƽ��ֵ��t2-t1��/�� | ||
H2SO4 | NaOH | ƽ��ֵ | |||
1 | 26.2 | 26.0 | 26.1 | 30.1 | __________ |
2 | 27.0 | 27.4 | 27.2 | 31.3 | |
3 | 25.9 | 25.9 | 25.9 | 29.8 | |
4 | 26.4 | 26.2 | 26.3 | 31.4 | |
(2)������Ϊ0.50mol��L-1 NaOH ��Һ��0.50mol��L-1������Һ���ܶȶ���1g��cm-3���кͺ�������Һ�ı�����c =4.18J��g-1����-1�����к�����H=__________��(ȡС�����һλ)
(3)����ʵ����ֵ�����57.3kJ��mol-1��ƫ�����ƫ���ԭ�������__________������ĸ����
a.ʵ��װ�ñ��¡�����Ч����
b.��ȡNaOH��Һ�����ʱ���Ӷ���
c.�ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d.���¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�
���𰸡�0.05mol/(L��min) 0.8 400K��1MPa 4.0 66.9 kJ/mol b
��������
(1)�ȼ���ӷ�Ӧ��ʼ��ƽ��ʱN2�ķ�Ӧ���ʣ�Ȼ��������ʱȵ��ڻ�ѧ����ʽ�Ļ�ѧ�������ıȼ���v(NO)��
(2)��ҵ�ϴ�װ�ñȽ��ʺϵ��¶Ⱥ�ѹǿ��NOƽ��ת��������豸����ѹǿ��С���豸���Ͼ���Ч��ã�
II.(1)���ж��¶Ȳ����Ч�ԣ�Ȼ������¶Ȳ�ƽ��ֵ��
(2)�ȸ���Q=mc��T���㷴Ӧ�ų���������Ȼ�������H=-![]() kJ/mol�������Ӧ�ȣ�
kJ/mol�������Ӧ�ȣ�
(3)������H=-![]() kJ/mol ��Q=mc��T����ʵ����
kJ/mol ��Q=mc��T����ʵ����
I.(1)����ͼ1��֪���ӷ�Ӧ��ʼ��ƽ��ʱ����c(N2)=0.10mol/L����v(N2)=![]() =0.025mol/(L��min)�����ݷ���ʽ2NO(g)+2CO(g)
=0.025mol/(L��min)�����ݷ���ʽ2NO(g)+2CO(g)![]() N2(g)+2CO2(g)��֪v(NO)=2v(N2)=2��0.025mol/(L��min)=0.05 mol/(L��min)��
N2(g)+2CO2(g)��֪v(NO)=2v(N2)=2��0.025mol/(L��min)=0.05 mol/(L��min)��
���ݷ���ʽ��֪��ÿ��Ӧ����1mol/LN2����ͬʱ����2mol/LCO������4minĩ��c(N2)=0.10mol/L������CO�ı�Ũ��Ϊ0.20mol/L�����ڷ�Ӧ��ʼʱCO��Ũ��Ϊ1mol/L�����ƽ��ʱCO��Ũ��Ϊ1mol/L-0.20mol/L=0.80mol/L��
(2)��Ӧ��NO��ƽ��ת������ѹǿ���¶ȵĹ�ϵ��ͼ2��ʾ�������仯������֪����ҵ�ϴ�װ�ñȽ��ʺϵ��¶Ⱥ�ѹǿ��400 K��1 MPa��
II. (1)4���¶Ȳ�ֱ�Ϊ��4.0�棬4.1�棬3.9�棬5.1�棬����������ƫ��̫��Ҫ��ȥ�������¶Ȳ�ƽ��ֵΪ4.0�棻
(2)�ᡢ���кͷ�Ӧʵ����H++OH-=H2O��50mL 0.5mol/L NaOH��Һ��50mL0.5mol/Lϡ������Һ�����кͷ�Ӧ����ˮ�����ʵ��������ʵ����ٵ�NaOH������n(H2O)=0.05L��0.50mol/L=0.025mol����Һ������Ϊ��100mL��1g/cm3=100g���¶ȱ仯��ֵΪ��T=4�棬������0.025molˮ�ų�������ΪQ=mc��T=100g��4.18J/��g�棩��4.0��=1672J����1.672kJ������ʵ���õ��к�����H=-![]() =66.9 kJ/mol��
=66.9 kJ/mol��
(3)a.װ�ñ��¡�����Ч�����õ�����ƫС���к��ȵ���ֵƫС��a����
b.��ȡNaOH��Һ�����ʱ���Ӷ������ᵼ�������������������ƫ�ų�������ƫ�ߣ��к��ȵ���ֵƫ��b��ȷ��
c.�ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У�����ɢʧ�϶࣬����¶�ƫ�ͣ��к��ȵ���ֵƫС��c����
d.�¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�Ӳ���ϡH2SO4���¶ȣ��������ʼ�¶�ƫ�ߣ���õ�����ƫС���к��ȵ���ֵƫС��d����
�ʺ���ѡ����b��

����Ŀ��ijѧ����Na2SO3��AgNO3�ڲ�ͬpH�µķ�Ӧ����̽����
��1�����Na2SO3��ҺpH��10��AgNO3��ҺpH��5��ԭ���ǣ������ӷ���ʽ��ʾ����___��
��2������pH��ʵ���¼���£�
ʵ����� | pH | ʵ������ |
a | 10 | ������ɫ�������Ժ��ܽ⣬��Һ���� |
b | 6 | ������ɫ������һ��ʱ�����δ�ܽ� |
c | 2 | ����������ɫ������һ��ʱ���������״�غ�ɫ����X |
�������ϵ�֪��
��.Ag2SO3����ɫ��������ˮ�����ڹ�����Na2SO3��Һ��
��.Ag2O���غ�ɫ��������ˮ���ܺ��ᷴӦ��
��ѧ���Բ����İ�ɫ������������ּ��裺
�ٰ�ɫ����ΪAg2SO3��
�ڰ�ɫ����ΪAg2SO4���Ʋ��������___��
��3��ȡb��c�а�ɫ����������Na2SO3��Һ�У������ܽ⡣��ͬѧ���ʵ��ȷ���˰�ɫ��������Ag2SO4��ʵ�鷽���ǣ���ȡAg2SO4��������__��Һ�У�δ�ܽ⡣
��4����c��X�˳���ϴ����Ϊȷ������ɣ�ʵ�����£�
��.��X�еμ�ϡ���ᣬ�����Ա仯��
��.��X�м������ŨHNO3����������ɫ���塣
��.�ֱ���Ba(NO3)2��BaCl2��Һ������з�Ӧ�����Һ��ǰ�������Ա仯�����߲�����ɫ������
��ʵ����Ŀ����___��
�ڸ���ʵ�����������X�ijɷ���___��
�ۢ��з�Ӧ�Ļ�ѧ����ʽ��___��
��5����ͬѧ�ۺ�����ʵ�飬��������X��ԭ���������Ե���ǿ����ϵ�Ļ�ԭ����ǿ��