��Ŀ����
����Ŀ����Ҫ����գ�
��1������������HOCH2CH2CHO_____�� ________��
________��
��2��ȼ��ij�л���A 1.50 g������1.12 L(��״��)CO2��0.05 mol H2O�����л���������Կ���������ܶ���1.04������л���ķ���ʽΪ_____��
��3����֪��Ȳ���ڴ��������¿ɷ���ż����Ӧ:2RC��CH![]() RC��C��C��CR+H2,��ΪGlaser��Ӧ���ش��������⣺
RC��C��C��CR+H2,��ΪGlaser��Ӧ���ش��������⣺
��֪: ����ת����ϵ�IJ���E��C16H10���Ľṹ��ʽ��_______����1 mol E�ϳ�1,4���������飬��������Ҫ��������____mol��
����ת����ϵ�IJ���E��C16H10���Ľṹ��ʽ��_______����1 mol E�ϳ�1,4���������飬��������Ҫ��������____mol��
���𰸡�3-�ǻ���ȩ �ڼ����ӣ�2-�����ӣ� CH2O ![]() 4
4
��������
��1���л��ﺬ��3��̼ԭ�ӣ������DZ�ȩ���ǻ�������3��̼ԭ���ϣ����ͷ��ǻ�������λ��ĸ��Ϊ���ӣ���������2��̼ԭ���ϣ�
��2�����л���������Կ���������ܶ���1.04��˵��Ħ������Ϊ29g/mol��1.04=30.16 g/mol��n(CO2)=![]() =0.05mol���л�����̼������m(C)=0.05mol��12g/mol=0.6g���������m(H)=0.05mol��2��1g/mol=0.1g��m(C)+ m(H)<1.5g��˵���л���A�к�����Ԫ�أ���m(O)=1.5g-0.6g-0.1g=0.8g������n(A):n(C):n(H): n(O)ȷ���л���ķ���ʽ��
=0.05mol���л�����̼������m(C)=0.05mol��12g/mol=0.6g���������m(H)=0.05mol��2��1g/mol=0.1g��m(C)+ m(H)<1.5g��˵���л���A�к�����Ԫ�أ���m(O)=1.5g-0.6g-0.1g=0.8g������n(A):n(C):n(H): n(O)ȷ���л���ķ���ʽ��
��3������Glaser��Ӧ�ķ�Ӧԭ��ȷ��E�Ľṹ��![]() �����������ӳɷ�Ӧ��ɵ�1,4���������飻
�����������ӳɷ�Ӧ��ɵ�1,4���������飻
��1���л��ﺬ��3��̼ԭ�ӣ������DZ�ȩ���ǻ�������3��̼ԭ���ϣ���˸��л��������Ϊ3-�ǻ���ȩ�����ͷ��ǻ�������λ��ĸ��Ϊ���ӣ���������2��̼ԭ���ϣ���˸��л��������Ϊ�ڼ����ӻ�2-�����ӣ�
��2���ɷ�����֪�л���A�к�����Ԫ�أ���m(O)=1.5g-0.6g-0.1g=0.8g��n(O)=![]() =0.05mol����n(A)=
=0.05mol����n(A)=![]() =0.05mol�����n(A):n(C):n(H): n(O)=0.05:0.05:0.1:0.05=1:1:2:1�����л���ķ���ʽΪCH2O��
=0.05mol�����n(A):n(C):n(H): n(O)=0.05:0.05:0.1:0.05=1:1:2:1�����л���ķ���ʽΪCH2O��
��3����Ȳ���ڴ������ڷ���Glaser��Ӧ�����ɵ�E�Ľṹ��ʽΪ![]() �� 1,4����������ĽṹΪ
�� 1,4����������ĽṹΪ![]() ,
,![]() ��4molH2������Ӧ�ɵ�
��4molH2������Ӧ�ɵ�![]() ��
��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ���о�![]() ��
��![]() ��
��![]() �ȵĴ��������Ի�����������Ҫ���塣
�ȵĴ��������Ի�����������Ҫ���塣
��1����ѧ�������о����ô�������β���е�NO��COת���![]() ��
��![]() ���䷴ӦΪ��
���䷴ӦΪ��![]()
![]()
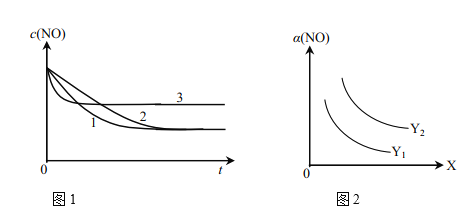
��Ϊ���о���������Ը÷�Ӧ��Ӱ�죬�����±�����ʵ�飬��ò�ͬʱ��NO��Ũ�ȣ�c����ʱ��仯��������ͼ1��ʾ��1��2��3������ʵ����������________������֪��ʹ�õ���������ʱ����������ȱ���������ѧ��Ӧ���ʡ���
ʵ���¶�NO��ʼŨ��O��ʼŨ�ȴ����ȱ��������������ţ��棩
ʵ�� ��� | �¶� ���棩 | NO��ʼŨ��
| CO��ʼŨ��
| �����ȱ����
| �������� ��g�� |
�� | 280 |
|
| 82 | 50 |
�� | 280 |
|
| 124 | 50 |
�� | 350 |
|
| 124 | 50 |
��ͼ2��ʾNO��ƽ��ת���ʣ�a�����¶ȡ�ѹǿ�仯��ʾ��ͼ��X��ʾ����________��������________��Y��ʾ����________����Y1________Y2������>������<������
��2��һ���¶��£���![]() ��
��![]() �������1��2�����ܱ������з�����Ӧ
�������1��2�����ܱ������з�����Ӧ![]() ���ﵽƽ��ʱ
���ﵽƽ��ʱ![]() ���������Ϊ25�����÷�Ӧ��ƽ�ⳣ��
���������Ϊ25�����÷�Ӧ��ƽ�ⳣ��![]() ________��
________��
��3������ԭ��ط�Ӧ��ʵ��![]() ���������ܷ�ӦΪ
���������ܷ�ӦΪ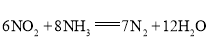 ���������ҺΪ���ԡ�����һ��ʱ��õ�ظ�����������ҺpH________�����������������С���������������������缫��ӦʽΪ________��
���������ҺΪ���ԡ�����һ��ʱ��õ�ظ�����������ҺpH________�����������������С���������������������缫��ӦʽΪ________��
����Ŀ��I.2017��5��5�գ��й��ܰ��չ��ʱ����Ƶ�ӵ������֪ʶ��Ȩ�Ĵ��Ϳͻ�C-919���Ϻ��ֶ������ɣ���ѧ����ʵ�������о������ô��������ɻ�β���е�NO��COת���CO2��N2�ķ�ӦΪ2NO(g)+2CO(g)![]() N2(g)+2CO2(g)��H<0��
N2(g)+2CO2(g)��H<0��
(1)����1mol NO��2mol COͨ��2L�ĺ����ܱ������У���һ�������·���������Ӧ����Ӧ�����ɵ�N2�����ʵ���Ũ����ʱ��ı仯�����ͼ1��ʾ����NO�ӷ�Ӧ��ʼ��ƽ��ʱ��ƽ����Ӧ����v(NO)=__________��4minĩCO��Ũ��Ϊ__________mol��L-1��
(2)��֪������Ӧ��NO��ƽ��ת������ѹǿ���¶ȵĹ�ϵ��ͼ2��ʾ����ҵ�ϴ�װ���бȽ��ʺϵ��¶Ⱥ�ѹǿ��__________

II.ȡ50 mL0.50mol��L-1NaOH��Һ��50 mL��0.50mol/L������Һ�����к��Ȳⶨʵ�飬ʵ���������±���
(1)����д���еĿհף�
�¶� ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�¶�t2/�� | �¶Ȳ�ƽ��ֵ��t2-t1��/�� | ||
H2SO4 | NaOH | ƽ��ֵ | |||
1 | 26.2 | 26.0 | 26.1 | 30.1 | __________ |
2 | 27.0 | 27.4 | 27.2 | 31.3 | |
3 | 25.9 | 25.9 | 25.9 | 29.8 | |
4 | 26.4 | 26.2 | 26.3 | 31.4 | |
(2)������Ϊ0.50mol��L-1 NaOH ��Һ��0.50mol��L-1������Һ���ܶȶ���1g��cm-3���кͺ�������Һ�ı�����c =4.18J��g-1����-1�����к�����H=__________��(ȡС�����һλ)
(3)����ʵ����ֵ�����57.3kJ��mol-1��ƫ�����ƫ���ԭ�������__________������ĸ����
a.ʵ��װ�ñ��¡�����Ч����
b.��ȡNaOH��Һ�����ʱ���Ӷ���
c.�ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ���
d.���¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�ֱ�ӲⶨH2SO4��Һ���¶�