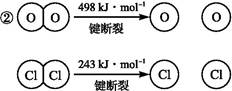
��Ŀ����
�����֣�ʳ���еĿ�����������軯�أ��仯ѧʽΪK4[Fe(CN)6]��3H2O��42.2 g K4[Fe(CN)6]��3H2O��Ʒ������ˮ���̵���������(��Ʒ�������¶ȵı仯����)����ͼ��ʾ��
�Իش��������⣺
(1)��ȷ��150 ��ʱ�������ʵĻ�ѧʽ_____________��
(2)��������֪����Ȼ�����軯���������Ժܵͣ�����ˮ��Һ���ᷴӦ�ų��������軯��(HCN)���壻�����軯�ؼ�����һ���¶�ʱ�ֽܷ�����軯��(KCN)���ݴ��жϣ����ʳƷʱӦע�������Ϊ_____________________________________________________��
(3)��Fe2����Fe3���Ĵ������£���ʵ��2SO2��O2��2H2O��2H2SO4��ת������֪����SO2�ķ���ͨ�뺬Fe2����Fe3������Һ��ʱ������һ����Ӧ�����ӷ���ʽΪ4Fe2����O2��4H����4Fe3����2H2O������һ����Ӧ�����ӷ���ʽΪ__________________________��
(4)��֪Fe(OH)3���ܶȻ�����Ksp��1.1��10��36������ʱ��FeCl3��Һ�еμ�NaOH��Һ������ҺpHΪ3ʱ��ͨ������˵��Fe3���Ƿ������ȫ_____________��(��ʾ����ij����Ũ��С��10��5 mol��L��1ʱ������Ϊ�����ӳ�����ȫ)
(ÿ�գ��֣���ͬ�����8��)(1)K4[Fe(CN)6]
(2)���������������һ�������������¶Ȳ�����400 ��
(3)2Fe3����SO2��2H2O��2Fe2����SO42-��4H����
(4)c(Fe3��)��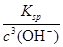 ��1.1��10��3 mol��L��1��1��10��5 mol��L��1����Fe3��û�г�����ȫ
��1.1��10��3 mol��L��1��1��10��5 mol��L��1����Fe3��û�г�����ȫ
���������������1��42.2 g K4[Fe(CN)6]��3H2O���ʵ�����42.2g��422g/mol��0.1mol�����нᾧˮ�����ʵ�����0.3mol��������0.3mol��18g/mol��5.4g������ͼ���֪����Ӧ���е�150��ʱ������ٵ�������42.2g��36.8g��5.4g����˵����ʱ���ٵ��������ǽᾧˮ����������˹���Ļ�ѧʽΪK4[Fe(CN)6]��
��2�����������軯��ˮ��Һ���ᷴӦ�ų��������軯��(HCN)���壬�������軯�ؼ�����һ���¶�ʱ�ֽܷ�����軯��(KCN)��������ʹ��ʱӦ��ע����������������һ�������������¶Ȳ�����400 �档
��3��������Fe2����Fe3���Ĵ������£���ʵ��2SO2��O2��2H2O��2H2SO4��ת��������ݷ���ʽ4Fe2����O2��4H����4Fe3����2H2O��֪��ǰ��ȥ�����õ���һ����Ӧ�����ӷ���ʽΪ2Fe3����SO2��2H2O��2Fe2����SO42-��4H����
��4������ҺpHΪ3ʱ������Һ��OH��Ũ����10��11mol/L�����ʱ��Һ��c(Fe3��)��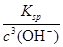 ��1.1��10��3 mol��L��1��1��10��5 mol��L��1����Fe3��û�г�����ȫ��
��1.1��10��3 mol��L��1��1��10��5 mol��L��1����Fe3��û�г�����ȫ��
���㣺�������ʻ�ѧʽȷ���ļ��㡢��������Ӧ�á�������ԭ��Ӧ����ʽ���ж��Լ��ܶȻ������ļ���

 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�A��B��C��D��E��F��G�������ʼ������ͼ��ʾ��ת����ϵ������A��B��D��G����ͬ��Ԫ�ء�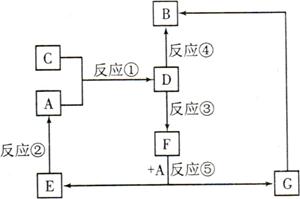
��֪��
AΪ�������ʣ�BΪ���ɫ���壬EΪ�ܶ���С�����壬GΪdz��ɫ����Һ��
D��ˮ��ҺΪ��ɫ��Һ��������������Һ��Ӧ���ɲ�����ϡ����İ�ɫ������
��ˮ��Һ��D�ܽ�ij����������ΪF��F�Ǻ�������Ԫ�صĻ����
��ش��������⣺
��1������C���ʵ�Ԫ�������ڱ��е�λ���� ���ڶ���������Ԫ���У���Ԫ����������Ԫ�ص�ԭ�Ӱ뾶�Ӵ�С��˳���� (��Ԫ�ط��ű�ʾ)��
��2��D��ˮ��Һ�� �ԣ��������ӷ���ʽ����ԭ�� ��
��3��������Ӧ�������û���Ӧ���� (�����)��
��4����Ӧ��(��D��ij������������ΪF)�����ӷ���ʽ�� ��
��5��������C��������ʵ�顣��֪������Ӧ�����У�ÿ����0.1mol KI��ת�Ƶĵ�����ԼΪ3.612��1023�����밴��Ҫ����գ�
| ʵ�鲽�� | ʵ������ | д���ӷ���ʽ |
| ����������ͨ�����KI��Һ | ��Һ������ ɫ | |
| ����ͨ������ | ��Һ�����ɫ | |
(1)ij��ɫϡ��ҺX�У����ܺ����±����������е�ij���֡�
| ������ | CO32����SiO32����AlO2����Cl�� |
| ������ | Al3����Cu2����Mg2����NH4+��Na�� |
��ȡ����Һ�����������м���ij�Լ�Y���������������ʵ���(n)������Լ�Y�����(V)�Ĺ�ϵ��ͼ��ʾ��
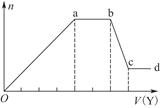
����Y�����ᣬ����Һ�к��еĽ�����������_________________________________��
ab�η�����Ӧ�������ӷ���ʽΪ___________________________________
����Oa����Y��Һ��Ӧ�����ӵ����ʵ���֮��Ϊ__________[Ҫ�������ӷ��ţ���n(Na��)]��
����Y��NaOH��Һ����bc�η�Ӧ�����ӷ���ʽΪ______________________________
�����������ӵ�ˮ�����أ�����H����OH����Ӱ�죬����Һ��ֻ����4�����ӣ������ǵ����Ӹ�����Ϊ___________________________________[����������ǰ���������ں���ǰ���ͼ��ں��˳������]��
(2)��Ϊ�ڢ�A��Ԫ�أ����ĵ��ʺͻ�������ijЩ���ʵĻ�ѧ����������������֮������֪��Ԫ�ؾ����������ʣ�
Sn4����Sn=2Sn2����
2Sn2����O2��4H��=2Sn4����2H2O��
2H����SnO22��
 Sn(OH)2
Sn(OH)2 Sn2����2OH����
Sn2����2OH�����Իش�
�����������ᣬ����Ӧ�����Һ��ͨ���������йط�Ӧ������������Ӧ�仯����д���йط�Ӧ�����ӷ���ʽ��______________________________________��
�ڽ�������Һ���ɺ�����������ù��壬�仯����������FeCl3��Һ��Ӧ�ı仯�������õ��Ĺ���������(����ʽ)__________��
��������SnCl2��Һ������ļ���Һ��Ӧ�ķ�����Sn(OH)2, �ü���__________��
A��B��C��D�������ʾ�Ϊ����������ɵĿ����Ի����������������ʵ����ӣ����Ӳ����ظ���ϣ��У�
| ������ | Na+��Al3+��Ba2+��NH4+ |
| ������ | Cl����OH����CO32����SO42�� |
�ֱ�ȡ�������ʽ���ʵ�飬ʵ�������¢�B��Һ�ֱ���C��D��ϣ����а�ɫ�������ɢڽ�A��Һ��ε���C��Һ�У��г������ɣ������μ�A��Һʱ����������ֱ����ȫ��ʧ��A��D���ֹ��������������ɣ���������ʹʪ��ĺ�ɫʯ����Һ��������ʯī�缫���B��Һ���������ϲ���һ���д̼�����ζ������
�ش��������⣺
��1��A�����������Ӻ�C���������ӵİ뾶��С____��______�������ӷ��ţ���B��������������________
��2��C��Һ��___�ԣ�����ԡ����ԡ�������ԭ����__________________
�������ӷ���ʽ���ͣ���D�Ļ�ѧʽ��____________
��3����PtΪ�缫���1L0.1mol/LB��ˮ��Һ������·��ͨ��0.1mol����ʱ��
��Һ��pHΪ_______�����������Һ������䣩�������ĵ缫��ӦʽΪ��_____
��4����������������������ͨ��A��Һ����ǡ����ȫ��Ӧʱ������Һ�и�����
Ũ���ɴ�С������˳��Ϊ__________________________
KMnO4��һ����Ҫ����������
��1����������������KMnO4�������Ի���ǿ�������ữKMnO4��Һ������Լ��� ��
a������ b������ c������
�ڽ�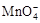 ����C2O42?�����ӷ���ʽ����������
����C2O42?�����ӷ���ʽ����������
��MnO4? +��C2O42?+�� ==��Mn2+ +��CO2��+�� ��
��2��ijͬѧΪ��̽��KMnO4��Һ��Na2C2O4�������ƣ���Һ�ķ�Ӧ���̣���������ʵ�飺
������100 mL 0.0400 mol��L-1��Na2C2O4��Һ�����õ�������ƽ��ҩ�ס��ձ�����Ͳ���������������⣬�������õ��IJ��������� ��
�ڽ�KMnO4��Һ��ε���һ�����������Na2C2O4��Һ�У��¶���ͬ��������������¼���������£�
| ����KMnO4��Һ�Ĵ��� | KMnO4��Һ��ɫ��ȥ�����ʱ�� |
| �ȵ����1�� | 60 s |
| ��ɫ���ٵ����2�� | 15 s |
| ��ɫ���ٵ����3�� | 3 s |
| ��ɫ���ٵ����4�� | 1 s |
�����KMnO4��Һ��ɫʱ��仯�Ŀ���ԭ�� ��
�������ƺõ�0.040 0 mol��L-1��Na2C2O4��Һ���궨ijKMnO4��Һ��Ũ�ȡ�ÿ��ȷ��ȡ25.00 mLNa2C2O4��Һ��Ȼ�����ữ���KMnO4��Һ�ζ����ζ����ηֱ����ĵ�KMnO4��Һ�������20.00 mL��19.80 mL��20.20 mL����KMnO4��Һ��Ũ��Ϊ ��
 2Cl2+2H2O,��ʵ���ȵ�ѭ�����á�
2Cl2+2H2O,��ʵ���ȵ�ѭ�����á�