��Ŀ����
(1)ij��ɫϡ��ҺX�У����ܺ����±����������е�ij���֡�
| ������ | CO32����SiO32����AlO2����Cl�� |
| ������ | Al3����Cu2����Mg2����NH4+��Na�� |
��ȡ����Һ�����������м���ij�Լ�Y���������������ʵ���(n)������Լ�Y�����(V)�Ĺ�ϵ��ͼ��ʾ��
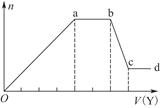
����Y�����ᣬ����Һ�к��еĽ�����������_________________________________��
ab�η�����Ӧ�������ӷ���ʽΪ___________________________________
����Oa����Y��Һ��Ӧ�����ӵ����ʵ���֮��Ϊ__________[Ҫ�������ӷ��ţ���n(Na��)]��
����Y��NaOH��Һ����bc�η�Ӧ�����ӷ���ʽΪ______________________________
�����������ӵ�ˮ�����أ�����H����OH����Ӱ�죬����Һ��ֻ����4�����ӣ������ǵ����Ӹ�����Ϊ___________________________________[����������ǰ���������ں���ǰ���ͼ��ں��˳������]��
(2)��Ϊ�ڢ�A��Ԫ�أ����ĵ��ʺͻ�������ijЩ���ʵĻ�ѧ����������������֮������֪��Ԫ�ؾ����������ʣ�
Sn4����Sn=2Sn2����
2Sn2����O2��4H��=2Sn4����2H2O��
2H����SnO22��
 Sn(OH)2
Sn(OH)2 Sn2����2OH����
Sn2����2OH�����Իش�
�����������ᣬ����Ӧ�����Һ��ͨ���������йط�Ӧ������������Ӧ�仯����д���йط�Ӧ�����ӷ���ʽ��______________________________________��
�ڽ�������Һ���ɺ�����������ù��壬�仯����������FeCl3��Һ��Ӧ�ı仯�������õ��Ĺ���������(����ʽ)__________��
��������SnCl2��Һ������ļ���Һ��Ӧ�ķ�����Sn(OH)2, �ü���__________��
(1)��Na����CO32����2H��=H2O��CO2��
n(SiO32��)��n(AlO2��)��11��2
��Al(OH)3��OH��=AlO2����2H2O
N(Al3��)��N(Mg2��)��N(NH4+)��N(Cl��)��2��1��4��12
(2)��Sn��2H��=Sn2����H2��
Sn2����Cl2=Sn4����2Cl��
��SnO2����NH3��H2O
����

��.�������(K2FeO4)�Ǽ��õ������������и�Ч���������ã�Ϊһ�����ͷ��ȸ�Ч�������������������������£�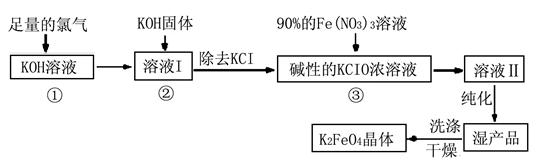
��ͬ���������⡣
��1��д����KOH��Һ��ͨ������Cl2������Ӧ�����ӷ���ʽ ��
��2������ҺI�м���KOH�����Ŀ���� (ѡ�����)��
| A��Ϊ��һ����Ӧ�ṩ���ԵĻ��� |
| B��ʹKClO3ת��ΪKClO |
| C������ҺI�й�����Cl2������Ӧ�����ɸ����KClO |
| D��KOH�����ܽ��ų��϶����������������߷�Ӧ���ʺ�KClO�Ĵ��� |
��4�����������һ�����Ͷ��ε�أ����ҺΪ����Һ���䷴ӦʽΪ��
3Zn(OH)2+2Fe(OH)3+4KOH
 3Zn+2K2FeO4+8H2O��
3Zn+2K2FeO4+8H2O���ŵ�ʱ��صĸ�����ӦʽΪ ��
��.��(N2H4)�ֳ���������һ�ֿ�ȼ�Ե�Һ�壬�������ȼ�ϡ�
��5��д���·��ӵĵ���ʽ ��
��6��������N2O4��Ӧ��2N2H4(g)��N2O4(g)=3N2(g)��4H2O(g) ��H����1076.7 kJ/mol��
��֪��N2(g)��2O2(g)=N2O4(g)����H����8.7 kJ/mol�� д������O2��Ӧ����N2��H2O(g)���Ȼ�ѧ����ʽ ��
�������ȣ�ClO2����Ŀǰ�����Ϲ��ϵĵ��Ĵ���Ч����������������һ�ֻ���ɫ�����壬������ˮ��
��.��1��ClO2����KClO3��H2SO4���ڵ���������Na2SO3��Ӧ�Ƶá���÷�Ӧ�����������뻹ԭ��������ʵ���֮����________��
��.ʵ����Ҳ����NH4Cl�����ᡢNaClO2���������ƣ�Ϊԭ���Ʊ�ClO2�����������£�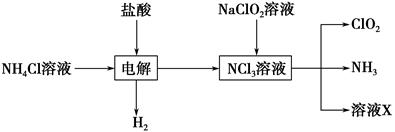
��2��д�����ʱ������Ӧ�Ļ�ѧ����ʽ��________________________________��
��3����ȥClO2�е�NH3��ѡ�õ��Լ���________��������ţ�
| A������ʳ��ˮ | B����ʯ�� |
| C��Ũ���� | D��ˮ |
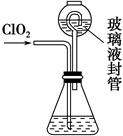
��װ���в���Һ��ܵ�������______________________________________��
����д��������������������⻯����Һ��Ӧ�����ӷ���ʽ__________________________��
�۵ζ��յ��������_______________________________________________��
�ܲ��ͨ��ClO2������m��ClO2����________�����ú�c��V�Ĵ���ʽ��ʾ��
��5����ClO2������������ˮ��pHΪ5.5��6.5��������һ���������岻���������������ClO2-��2001���ҹ��������涨������ˮ��ClO2-����Ӧ������0.2 mg��L��1��������ˮ��ClO2-�ĺ������꣬�������м���������ij��ԭ�����÷�Ӧ������������________���ѧʽ�����䷢����Ӧ�����ӷ���ʽΪ_________________________________________________________________��
���ֺ��ؽ��У�����ˮ��ȫռ�м�Ϊ��Ҫ�ĵ�λ��ij�о�С����ȡ��������Ⱦ��ˮԴ���з�����������������ʵ����Ϣ������һ������Ⱦ��ˮԴ����A��B�������ʣ�һ������C��D�������ʣ�һ������E���ʣ�A��B��C��D��E���ֳ��������ﶼ�����±��е������γɵģ�
| ������ | K����Na����Cu2����Al3�� |
| ������ | SO42����HCO3����NO3����OH�� |
Ϊ�˼�������������ֱ��������ʵ�飬�����ǣ�
�ٽ���������ˮ��DΪ��ɫ��Һ��������Ϊ��ɫ��Һ��
�ڽ�E��Һ���뵽C��Һ�У����ְ�ɫ�����������μӳ����ܽ⡣
�۽�����ɫ��Ӧʵ�飬ֻ��B��C���м����ӡ�
���ڸ���Һ�м���Ba(NO3)2��Һ���ټ������ϡ���ᣬA�зų���ɫ���壬C��D�в�����ɫ������
�ݽ�B��D����Һ��ϣ�δ���������������ɡ�
��������ʵ��������д���пհף�
(1)д��B��C��D�Ļ�ѧʽ��B________��C________��D________��
(2)����1 mol A����Һ�뺬1 mol E����Һ��Ӧ�����ɣ����õ�һ�ֻ�����û�����Ϊ________��
(3)д��ʵ��ڷ�����Ӧ�����ӷ���ʽ________________________________________
(4)C��������ˮ���������ӷ���ʽ��ʾ�侻ˮԭ��_______________________________
����ع㷺Ӧ���ڻ�϶�������ϵͳ���缫������Ni��OH��2��̿�ۡ���������Ϳ�����������Ƴɣ����ڵ��ʹ�ú�缫���϶Ի�����Σ����ij��ȤС��Ըõ�ص缫���Ͻ�����Դ�����о�������Ƴ����ʵ������ͼ
��֪����NiCl2������ˮ��Fe3+��������Ni2+
��ij�¶���һЩ�������������Ksp����������������pH�����ʾ��
| M��OH��n | Ksp | pH | |
| ��ʼ���� | ������ȫ | ||
| Al��OH��3 | 1.9��10-33 | 3.43 | 4.19 |
| Fe��OH��3 | 3.8��10-38 | 2.53 | 2.94 |
| Ni��OH��2 | 1.6��10-14 | 7.60 | 9.75 |
�ش��������⣺
��1�����ݱ������жϲ������������������ �ͳ����� ���ѧʽ������pH1 pH2�������������=�����������������ֳ������������� ��
A��pH��ֽ B��ʯ��ָʾ�� C��pH��
��2����֪�ܽ�ȣ�NiC2O4��NiC2O4?H2O��NiC2O4?2H2O����Ӧ�۵Ļ�ѧ����ʽ�� ���ڢ۲���Ӧ���˳���ʱ��Ҫ�IJ��������� ��������ʱ������Һ�����������ǣ���ʵ������ĽǶȸ������ֿ��ܵ�ԭ�� �� ��
��3������������Ӧ����������EΪ ����֤��������Լ�Ϊ ��
��4����д����Ӧ�����ӷ���ʽ_____________________________________________
ij��ҵ��ˮ�����±��е�ijЩ���ӣ��Ҹ������ӵ����ʵ���Ũ����ȣ���Ϊ0.1 mol/L(����ֵ����ˮ�ĵ��뼰���ӵ�ˮ��)��
| ������ | K����Ag����Mg2����Cu2����Al3����NH4+ |
| ������ | Cl����CO32����NO3����SO42����SiO32����I�� |
��ͬѧ��̽����ˮ����ɣ�����������ʵ�飺
��.ȡ����ɫ��Һ5 mL���μ�һ�ΰ�ˮ�г������ɣ��������������ӡ�
��.�ò�˿պȡ��Һ���ڻ��������գ�����ɫ�ܲ����۲죬����ɫ���档
��.��ȡ��Һ����������ᣬ����ɫ�������ɣ�����ɫ������������ɺ���ɫ��
��.��������õ���Һ�м���BaCl2��Һ���а�ɫ�������ɡ�
���ƶϣ�
(1)�ɢ��жϣ���Һ��һ�������е���������____________��
(2)���м�������������ɫ��������ӷ���ʽ��
___________________________________________________________��
(3)��ͬѧ����ȷ��ԭ��Һ��������������________����������________�����ݴ��Ʋ�ԭ��ҺӦ�ó�_______________________________________________�ԣ�ԭ����_________________________________(�������ӷ���ʽ˵��)��
(4)��ȡ100 mLԭ��Һ������������NaOH��Һ���˹������漰�����ӷ���ʽΪ__________________________________________________________��
��ַ�Ӧ����ˣ�ϴ�ӣ����ճ��������أ��õ��Ĺ�������Ϊ________g��
 Fe2����SO42��
Fe2����SO42��
