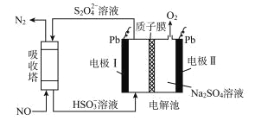
��Ŀ����
����Ŀ��CO2�Ļ����������ǿ�ѧ���о����ȵ���⣬������CH4��CO2�Ʊ����ϳ�������CO��H2���������Ʊ��״��������ѡ���̼ϩ����ȼ�ϲ�Ʒ��
I���ƺϳ���
��ѧ������Ʊ����ϳ�������Ӧ���̷�������
��Ӧ�٣�CH4��g��![]() C��ads��+2H2 ��g�� ������Ӧ��
C��ads��+2H2 ��g�� ������Ӧ��
��Ӧ�ڣ�C��ads��+ CO2��g��![]() 2CO��g�� ���췴Ӧ��
2CO��g�� ���췴Ӧ��
������Ӧ��C��ads��Ϊ�����Ի���̿����Ӧ���̵������仯��ͼ��

��1��CH4��CO2�Ʊ����ϳ��������Ȼ�ѧ����ʽΪ_________�������仯ͼ�У�E5+E1_________E4+E2������������������������������
II����ˮ����
�������ϳ������ϳɼ״��״���ˮ�Ƶö����ѵķ�ӦΪ��
2CH3OH��g��![]() CH3OCH3��g�� + H2O��g�� ��H�������ʷ���ʽΪ��v��= k����c2��CH3OH����v��=k����c��CH3OCH3����c��H2O����k����k��Ϊ���ʳ�����ֻ���¶��йء����������ϣ�������Ӧƽ��״̬�´��ڼ���ʽ��lnKc = 2.205+
CH3OCH3��g�� + H2O��g�� ��H�������ʷ���ʽΪ��v��= k����c2��CH3OH����v��=k����c��CH3OCH3����c��H2O����k����k��Ϊ���ʳ�����ֻ���¶��йء����������ϣ�������Ӧƽ��״̬�´��ڼ���ʽ��lnKc = 2.205+![]() ��KcΪ��ѧƽ�ⳣ����T Ϊ����ѧ�¶ȣ���λΪK����
��KcΪ��ѧƽ�ⳣ����T Ϊ����ѧ�¶ȣ���λΪK����
��2����Ӧ�ﵽƽ��������¶ȣ�k������ı���_________ k������ı���������������������������������
��3��ij�¶��£��÷�Ӧƽ�ⳣ��KcΪ200�������ܱ������м���һ���� CH3OH����Ӧ��ijʱ�̲�ø���ֵ����ʵ������£�
���� | CH3OH | CH3OCH3 | H2O |
���ʵ���/mol | 0.4 | 0.4 | 0.4 |
��ʱ�����淴Ӧ���ʵĴ�С��v�� ____v�� ������������ ������������������
��4��500K�£����ܱ������м���һ�����״� CH3OH����Ӧ����ƽ��״̬ʱ����ϵ��CH3OCH3��g�������ʵ�������Ϊ_________�����ţ���
A ��![]() B
B ![]() C ��
C ��![]() D ��ȷ��
D ��ȷ��
���𰸡�CH4(g)+CO2(g)![]() 2CO(g)+2H2(g)��H=+(E3-E1)kJmol-1 < < > C
2CO(g)+2H2(g)��H=+(E3-E1)kJmol-1 < < > C
��������
I����1����ͼ���֪��CH4��CO2�Ʊ����ϳ��������Ȼ�ѧ����ʽΪ��CH4(g)+CO2(g)![]() 2CO(g)+2H2(g)��H=+(E3-E1)kJmol-1����Ӧ��Ϊ����Ӧ����Ӧ��Ϊ�췴Ӧ����˿�֪��Ӧ�ٵĻ�ܴ��ڷ�Ӧ�ڵĻ�ܣ���E4-E1>E5-E2����E5+E1<E4+E2��
2CO(g)+2H2(g)��H=+(E3-E1)kJmol-1����Ӧ��Ϊ����Ӧ����Ӧ��Ϊ�췴Ӧ����˿�֪��Ӧ�ٵĻ�ܴ��ڷ�Ӧ�ڵĻ�ܣ���E4-E1>E5-E2����E5+E1<E4+E2��
II����2���¶����ߣ�lnKC��С��KC��С��˵�������¶ȣ�ƽ�������ƶ����������¶ȣ�k������ı���С��k������ı�����
��3����ʱ��Ũ����![]() <KC����˷�Ӧ������У�v��>v����
<KC����˷�Ӧ������У�v��>v����
��4��500K�£�lnKc = 2.205+![]() =3.21��KC=e3.21=24.78������ijһʱ��c(CH3OH)= c(CH3OCH3)= c(H2O)=amol/L����ʱH3OCH3(g)�����ʵ�������Ϊ
=3.21��KC=e3.21=24.78������ijһʱ��c(CH3OH)= c(CH3OCH3)= c(H2O)=amol/L����ʱH3OCH3(g)�����ʵ�������Ϊ![]() ����Ũ����
����Ũ����![]() <KC����˷�Ӧ������У��ﵽƽ��ʱ��CH3OCH3(g)�����ʵ�����������
<KC����˷�Ӧ������У��ﵽƽ��ʱ��CH3OCH3(g)�����ʵ�����������![]() ��
��

 ��ĩ100�ִ��غ�������ϵ�д�
��ĩ100�ִ��غ�������ϵ�д� Сѧ�������Ծ�ϵ�д�
Сѧ�������Ծ�ϵ�д�����Ŀ����������������Ҫ�ľ�ϸ�����Լ�������������ˮ����ʳ���㾫��ʵ�����Ʊ��������£�

�Լ�����������±���
������ | �Ҵ� | ���������� | |
������״ | ��ɫ��״���� | ��ɫҺ�� | ��ɫ��Һ�� |
�е�/�� | 249.0 | 78.0 | 212.6 |
��Է����� | 122 | 46 | 150 |
�ܽ��� | ����ˮ���������Ҵ������ѵ��л��ܼ� | ��ˮ����Ȼ��� | ��������ˮ��������ˮ���������Ҵ������� |
�ش��������⣺
��1��Ϊ���ԭ�ϱ�����Ĵ��ȣ��ɲ��õĴ�������Ϊ_________��
��2������ٵ�װ����ͼ��ʾ�����Ⱥͼг�װ������ȥ������һС������������B�п��������״�������ˮ������ˮ����ͭ���Ҵ�������Һ����������B�У�������C�м��� 12.2 g������ı����ᾧ�壬30 mL��ˮ�Ҵ���Լ0.5 mol����3 mLŨ���ᣬ�����ʯ���������У�������Ӧ1.5~2 h������A��������_________������C�з�ӦҺӦ����_________��ʽ���ȡ�
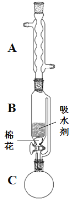
��3�����ŷ�Ӧ���У���Ӧ��ϵ��ˮ�ֲ��ϱ���Ч���룬����B����ˮ��������Ϊ_________��
��4����Ӧ������C�л��Һ���з����ᴿ������I��_________������II���õIJ������������ձ����_________��
��5����Ӧ����������н���ӦҺ������ˮ��Ŀ�ij����ܽ��Ҵ��⣬����_____�������Լ�XΪ_____����д��ѧʽ����
��6�����յõ����﴿Ʒ12.0 g��ʵ�����Ϊ_________ %��������λ��Ч���֣���
����Ŀ�������½�������ʵ�飬����ʵ��������������õ��Ľ�����ȷ����( )
ѡ�� | ʵ����������� | ���� |
A | ��ij��Һ���ȵμ�ϡ���ᣬ�ٵμ� | ����Һ��һ������ |
B | �� |
|
C | �� |
|
D | �� | ���ԣ� |
A.AB.BC.CD.D