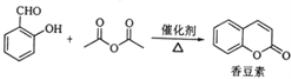
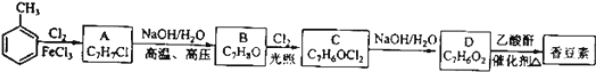
ЬтФПФкШн
ЁОЬтФПЁПРћгУДпЛЏбѕЛЏЗДгІНЋSO2зЊЛЏЮЊSO3ЪЧЙЄвЕЩЯЩњВњСђЫсЕФЙиМќВНжшЁЃ
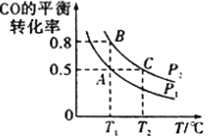
ЃЈ1ЃЉT1ЁцЪБЃЌдк2LУмБеШнЦїжаГфШы0.6molSO3ЃЌЭМ1БэЪОSO3ЮяжЪЕФСПЫцЪБМфЕФБфЛЏЧњЯпЁЃ

ЂйЦНКтЪБЃЌSO3ЕФзЊЛЏТЪЮЊ______ЃЈБЃСєвЛЮЛаЁЪ§ЃЉЃЛдкT1ЁцЯТЗДгІ2SO2(g)+O2(g) ![]() 2SO3(g) ЕФЦНКтГЃЪ§ЮЊ____________ЃЛШєЦфЫћЬѕМўВЛБфЃЌдк8minЪБбЙЫѕШнЦїЕФЬхЛ§жС1LЃЌдђn(SO3)ЕФБфЛЏЧњЯпЮЊ_______ЃЈЬюзжФИЃЉЁЃ
2SO3(g) ЕФЦНКтГЃЪ§ЮЊ____________ЃЛШєЦфЫћЬѕМўВЛБфЃЌдк8minЪБбЙЫѕШнЦїЕФЬхЛ§жС1LЃЌдђn(SO3)ЕФБфЛЏЧњЯпЮЊ_______ЃЈЬюзжФИЃЉЁЃ
ЂкЯТБэЮЊВЛЭЌЮТЖШ(T)ЯТЗДгІ2SO2(g)+O2(g) ![]() 2SO3(g) ЁїHЃМ0ЕФЛЏбЇЦНКтГЃЪ§ЃЈKЃЉЃК
2SO3(g) ЁїHЃМ0ЕФЛЏбЇЦНКтГЃЪ§ЃЈKЃЉЃК
T/Ёц | T2 | T3 |
K | 20.5 | 4.68 |
гЩДЫЭЦжЊЃЌЦфЫћЬѕМўЯрЭЌЃЌдкT1ЁЂT2ЁЂT3Ш§жжВЛЭЌЮТЖШЯТЃЌЗДгІДгПЊЪМжСДяЕНЦНКтЪБЫљашвЊЕФЪБМфзюГЄЕФЪЧ _____________ЁЃЃЈЬюЁАT1ЁБЁЂЁАT2ЁБЛђЁАT3ЁБЃЉ
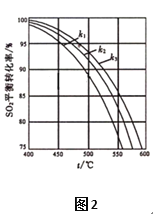
ЃЈ2ЃЉдкЬхЯЕКубЙЕФЬѕМўЯТНјааЗДгІЃК2SO2(g)+O2(g) ![]() 2SO3(g)ЃЌдСЯЦјжаSO2КЭO2ЕФЮяжЪЕФСПжЎБШЃЈkЃЉВЛЭЌЪБЃЌSO2ЕФЦНКтзЊЛЏТЪгыЮТЖШЃЈtЃЉЕФЙиЯЕШчЭМ2ЫљЪОЃКЭМжаk1ЁЂk2ЁЂk3ЕФДѓаЁЫГађЮЊ____________ЁЃ
2SO3(g)ЃЌдСЯЦјжаSO2КЭO2ЕФЮяжЪЕФСПжЎБШЃЈkЃЉВЛЭЌЪБЃЌSO2ЕФЦНКтзЊЛЏТЪгыЮТЖШЃЈtЃЉЕФЙиЯЕШчЭМ2ЫљЪОЃКЭМжаk1ЁЂk2ЁЂk3ЕФДѓаЁЫГађЮЊ____________ЁЃ
ЁОД№АИЁП 66.7% 2.5 c T2 k1>k2>k3
ЁОНтЮіЁП(1)дкШнЛ§ЮЊ2LУмБеШнЦїжаГфШы0.6molSO3ЃЌгЩЭМ1ЦНКтЪБSO3ЮяжЪЕФСПЮЊ0.2molЃЌ
2SO2(g)+O2(g) 2SO3(g)ЃЌ
Ц№ЪМСП(mol)0 0 0.6
БфЛЏСП(mol) 0.4 0.2 0.4
ЦНКтСП(mol) 0.4 0.2 0.2
ЂйSO3ЕФзЊЛЏТЪЮЊ=![]() ЁС100%Ёж66.7%ЃЛK=
ЁС100%Ёж66.7%ЃЛK=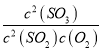 =
=![]() =2.5ЃЛЦфЫћЬѕМўВЛБфЃЌдк8minЪБбЙЫѕШнЦїЬхЛ§жС0.5LЃЌЦНКтЯђе§ЗДгІЗНЯђвЦЖЏЃЌn(SO3)БфДѓЃЌМДЮЊЭМЯёcЃЌЙЪД№АИЮЊЃК66.7%ЃЛ2.5ЃЛ cЃЛ
=2.5ЃЛЦфЫћЬѕМўВЛБфЃЌдк8minЪБбЙЫѕШнЦїЬхЛ§жС0.5LЃЌЦНКтЯђе§ЗДгІЗНЯђвЦЖЏЃЌn(SO3)БфДѓЃЌМДЮЊЭМЯёcЃЌЙЪД№АИЮЊЃК66.7%ЃЛ2.5ЃЛ cЃЛ
ЂквђЮЊЗДгІЮЊЗХШШЗДгІЃЌЮТЖШдНЕЭЃЌЦНКтдНЯђе§ЗДгІЗНЯђвЦЖЏЃЌЦНКтГЃЪ§дНДѓЃЌЫљвдЮТЖШзюЕЭЕФЮЊKзюДѓЕФЃЌМДT2ЃЌЙЪД№АИЮЊЃКT2ЃЛ
(2)ЯрЭЌЮТЖШКЭбЙЧПЯТЃЌKдНаЁЃЌбѕЦјХЈЖШдНДѓЃЌЦНКте§ЯђвЦЖЏЃЌдђЖўбѕЛЏСђЕФзЊЛЏТЪдНДѓЃЌдђk1ЃОk2ЃОk3ЃЌЙЪД№АИЮЊЃКk1ЃОk2ЃОk3ЁЃ

 ДКгъНЬг§ЭЌВНзїЮФЯЕСаД№АИ
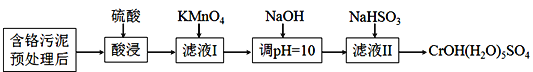
ДКгъНЬг§ЭЌВНзїЮФЯЕСаД№АИЁОЬтФПЁПФГКЌИѕЮлФржївЊКЌCr2O3ЁЂAl2O3ЁЂFe2O3ЕШЮяжЪЃЌИУЮлФрПЩвдгУЪЊЗЈЛиЪеРћгУЃЌСїГЬШчЯТЭМЫљЪОЃК
вбжЊЃКЪвЮТЯТВПЗжЧтбѕЛЏЮяЕФKspМћЯТБэ
Al(OH)3 | Fe(OH)3 | Cr(OH)3 | Mn(OH)2 | |
Ksp | 3ЁС10-34 | 4ЁС10-38 | 6ЁС10-31 | 4ЁС10-14 |
ЃЈ1ЃЉЫсНўЧАЃЌЖдКЌИѕЮлФрНјаадЄДІРэМДМгЫЎЪЊФЅГЩНЌЬхЃЌЪЊФЅЕФзїгУЪЧ_____________ЁЃ
ЃЈ2ЃЉЯђТЫвКIМгKMnO4бѕЛЏCr3+ЕФРызгЗНГЬЪНЪЧ________________________________;НЋCr3+бѕЛЏЕФФПЕФЪЧ____________________________________________________ЁЃ
ЃЈ3ЃЉЪвЮТЯТЃЌЕїpH=10ЪБЃЌ ![]() =_____ЃЛЕїШмвКpHВЛФмДѓгк10ЃЌРэгЩЪЧ_________ЁЃ
=_____ЃЛЕїШмвКpHВЛФмДѓгк10ЃЌРэгЩЪЧ_________ЁЃ
ЃЈ4ЃЉNaHSO3дкЗДгІжаЕФзїгУЪЧ___________ЃЛРэТлЩЯn(NaHSO3):n[CrOH(H2O)5SO4]жЎБШЪЧ_______________ЁЃ
ЃЈ5ЃЉNaHSO3ЙЬЬхдкПеЦјжавзБфжЪЃЌаДГіМьбщNaHSO3ЪЧЗёБфжЪЕФЗНЗЈ______________ЁЃ