��Ŀ����
5����1��ij�¶ȣ�t�棩ʱˮ��KW=1��10-13������¶ȣ� 25�棨�������������=��������������ˮ�ĵ��������ȵģ������¶�ʱˮ�ĵ���ƽ�������ƶ���Kw��������¶��µ�Kw��25��ʱ�����Ը��¶ȴ���25����¶���c��OH-��=1��10-7mol/L����Һ���� �ԣ���ᡱ������С�����������Һ��ֻ����HCl���ʣ�����ˮ�������c��H+��=1��10-7mol/L��2�������£���PH=3������aL�ֱ�������������Һ��ϣ������Һ�������ԣ�
��Ũ��Ϊ1.0��10-3mol/L�İ�ˮbL
��c��OH-��=1.0��10-3mol/L�İ�ˮcL
��c��OH-��=1.0��10-3mol/L��Ba��OH��2��ҺdL
��a��b��c��d֮���ɴ�С��˳����b��a=d��c
��3�������½�PH=5��H2SO4��Һ����ˮϡ�͵�1000������ϡ�ͺ�c��SO42-����c��H+���ı�ֵԼΪ1��20����c��H+����ͬ��HCl��CH3COOH��H2SO4����ˮϡ����ԭ����100����PHֵ�ɴ�С��˳���Ǣ٣���=�ۣ�
���� ��1��ˮ�ĵ��������ȷ�Ӧ�������¶ȴٽ�ˮ���룬����Һ�����ӻ�����������Һ���������c��H+����c��OH-��Ũ�ȵ���Դ�С��������������Һ�У������Ӽ���ȫ��������ˮ�ĵ��룬��������ȫ��������ˮ�ĵ��룻
��2��pH=3����������ʵ���Ũ��=1��10-3 mol/L���к���ͬ���ʵ��������ᣬ���Ũ��Խ�����õļ�Խ�٣�ע��������Һ�����������ӵ����ʵ���Ũ�Ⱥͼ��Ũ�Ȳ��ȣ�
��3����������ҺpH=5������ԭ��Һ��c��H+����ԭ��Һ��c��SO42-��=$\frac{1}{2}$c��H+����ϡ��1000������ʱ��Һ�ӽ����ԣ�������Ũ�Ȳ�����С��1��10-7mol/L��ֻ�����ӽ�1��10-7mol/L����ϡ����������������ʵ������䣬����ϡ�ͺ���Һ������������ʵ���Ũ�ȣ��ݴ˼����𣻴�����������ʼ�ˮϡ�ʹٽ����룬����ϡ�ͺ����������Ũ�������������������ӵ�Ũ��ֻ��С��
��� �⣺��1��ˮ�ĵ��������ȷ�Ӧ�������¶ȴٽ�ˮ���룬��ˮ�����ӻ���������ij�¶ȣ�T�棩ʱ��ˮ�����ӻ�����Kw=1��10-13��10-14������¶ȴ���25�棻
���¶���c��OH-��=1��10-7mol/L����Һ��c��H+��=$\frac{1{0}^{-13}}{1{0}^{-7}}$=10-6mol/L��c��H+����c��OH-��������Һ�����ԣ�������Һ�У������Ӽ���ȫ��������ˮ�ĵ��룬��������ȫ��������ˮ�ĵ��룬����Һ����ˮ�������c��OH-��=1��10-7mol/L����ˮ������������Ӻ���������Ũ����ȣ�����ˮ������������ӵ�Ũ��c��H+��=1��10-7mol/L��
�ʴ�Ϊ�����ڣ�ˮ�ĵ��������ȵģ������¶�ʱˮ�ĵ���ƽ�������ƶ���Kw��������¶��µ�Kw��25��ʱ�����Ը��¶ȴ���25�棻���ԣ�1��10-7mol/L��
��2��pH=3����������ʵ���Ũ��=1��10-3 mol/L��
���а�ˮ�����ʵ���Ũ����1��10-3 mol/L����һˮ�ϰ���������ʣ�ֻ�в��ֵ��룬���Ԣ��а�ˮ��Ũ�ȴ���1��10-3 mol/L���������������ӵ�Ũ����1��10-3 mol/L��
�����������������ǿ����ʣ������Ӻ������������к�ʱ��1��1�Ĺ�ϵ�������Ӻ����������ӵ�Ũ����ȣ�����a��d�������ȣ���a=d��
�ڵİ�ˮŨ�ȴ��ڢٵ�Ũ�ȣ��к���ͬ���ʵ��������ᣬ��ˮ��Ũ��Խ��ʹ�õİ�ˮ�����ԽС������c��b��
����Ͱ�ˮ��Ӧ���ɵ��Ȼ����ǿ�������Σ�ˮ���ʹ��Һ�����ԣ�Ҫ��ʹ��Һ�����ԣ���ˮ�����ʵ���Ӧ��������Ĵ�Щ��������Ũ�ȺͰ�ˮ��Ũ�����ʱ����ˮ�����bӦ������������a������Һ�����a��b��
�ڢ������������ӵ�Ũ����ȣ�һˮ�ϰ���һԪ������ʣ�����������ǿ����ʣ�����ˮ��Ũ�ȴ��ڢ�������������Ũ�ȣ��к���ͬ���ʵ�����������ʱ�������õİ�ˮ�����С�ڢ�����������Һ���������c��d=a��
����a��b��c��d�Ĺ�ϵb��a=d��c��
�ʴ�Ϊ��b��a=d��c��
��3��pHΪ5����Һ��������Ũ��Ϊ��c��H+��=1��10-5mol/L����������ӵ�Ũ��Ϊ��c��SO42-��=$\frac{1}{2}$c��H+��=$\frac{1}{2}$��1��10-5mol/L=5��10-6mol/L��
��Һϡ��1000����������Ũ�Ȳ�����С��1��10-7mol/L��ֻ�����ӽ�1��10-7mol/L�������������Ũ��Ϊ��c��SO42-��=5��10-6mol/L��$\frac{1}{1000}$=5��10-9mol/L��
����ϡ�ͺ���Һ��SO42-����Ũ����H+����Ũ�ȵı�ֵԼΪ��5��10-9mol/L��1��10-7mol/L=1��20��������������ʼ�ˮϡ�ʹٽ����룬����ϡ�ͺ����������Ũ�������������������ӵ�Ũ��ֻ��С������c��H+���ɴ�С��˳��Ϊ�٣���=�ۣ��ʴ�Ϊ��1��20���٣���=�ۣ�
���� ���⿼��������ʵĵ��룬��Ŀ�ѶȲ�����ע�����������ʵĵ����ص㣬���ݵ���ƽ�ⳣ��Ӧ�ã�

| A�� | �ù㷺pH��ֽ����ˮ��pH=4 | |
| B�� | ������1L��1mol/L��NaCl��Һ���ɽ�58.5gNaCl����1 Lˮ�� | |
| C�� | ������Һ����ʱ����������ƿ�̶Ȼ�ʹ������ҺŨ��ƫ�� | |
| D�� | �ô����������ۻ��������ƹ��� |
| A�� | ��С�մ�����θ�ᣨ��Ҫ�ɷ�Ϊ���ᣩ���ࣺHCO3-+H+=CO2��+H2O | |
| B�� | ����������Һ��������Һ��Ӧ��Ba2++SO42-=BaSO4�� | |
| C�� | �����ʯ��ˮ��ϡ���ᷴӦ��Ca��OH��2+2H+=Ca2++2H2O | |
| D�� | ������ʯ��ˮ��ͨ������������̼���壺CO2+OH-=HCO3- |
| A�� | ϡ H2SO4�����۷�Ӧ��2Fe+6H+=2Fe3++3H2�� | |
| B�� | ͭ����������Һ��Ӧ��Cu+Ag+=Cu2++Ag | |
| C�� | ̼��������ᷴӦ��CO32-+2H+=CO2��+H2O | |
| D�� | Ba��OH��2��CuSO4��Һ��Ӧ��Cu2++SO42-+Ba2++2OH-=BaSO4��+Cu��OH��2�� |
| A�� | ͭ��Ũ���ᷴӦ������ͭ | |
| B�� | ��ͭ���������Ҵ�����Ϊ��ȩ | |
| C�� | ��ϩ�ۺ�Ϊ����ϩ | |
| D�� | ���������¼�����������Ӧ��һ�ȼ��� |
| A�� | 0.9mol | B�� | 9mol | C�� | 1.8mol | D�� | 0.6mol |
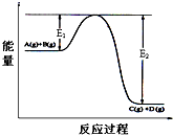
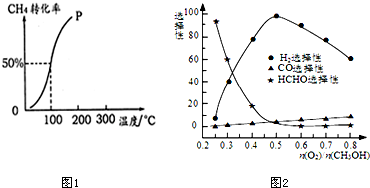 �״���Դ�ḻ���۸�������������淽�㣬��һ����Ҫ�Ļ���ԭ�ϣ�������Ҫ����;��Ӧ��ǰ������ҵ�����״��ij��÷����ǣ�CO��g��+2H2��g��?CH3OH��g����H=-90.8 kJ•mol-1
�״���Դ�ḻ���۸�������������淽�㣬��һ����Ҫ�Ļ���ԭ�ϣ�������Ҫ����;��Ӧ��ǰ������ҵ�����״��ij��÷����ǣ�CO��g��+2H2��g��?CH3OH��g����H=-90.8 kJ•mol-1