��Ŀ����
����Ŀ���軯�أ�KCN����һ���о綾�����ʣ������ʹ��ʱ����ע�ⰲȫ����֪��KCN+H2O2=KOCN+H2O���ش��������⣺
��1��OCN-����������Ԫ�صĵ�һ�����ܴӴ�С��˳��Ϊ_________����Ԫ�ط��ű�ʾ����ͬ�����縺�ԴӴ�С��˳��Ϊ________����̬��ԭ����Χ�����Ų�ʽΪ__________��
��2��H2O2�еĹ��ۼ�����Ϊ_______����Ҽ����м���) ��������ԭ�ӵ��ӻ��������Ϊ_________��������4��ԭ��______����ڡ����ڡ���ͬһ��ֱ���ϣ�H2O2������ˮ�����Ƕ��Ǽ��Է����⣬����Ϊ____________________��
��3����OCN-���Ϸ�ʽ��ͬ�һ�Ϊ�ȵ�����ķ���Ϊ________���ξ�һ����������OCN-��Ϊ�ȵ���������У���һ��Ԫ����ɵ���������____________��
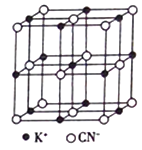
��4��KCN�ľ����ṹ��ͼ��ʾ��������K������λ��Ϊ_______�����侧������a=0.648nm����KCN ������ܶ�Ϊ_______g/cm3���г�����ʽ����
���𰸡� N>O>C O>N>C 2s22p3 ���� sp3 ���� H2O2������H2O��֮����γ���� CO2����N2O�� N3- 6 ![]()
����������1��C��N��O����ͬһ����Ԫ����ԭ���������μ�С��ͬһ����Ԫ�صĵ�һ����������ԭ���������������������A��Ĵ��ڵ���A��ģ��������һ�����ܴ�С˳����N��O��C��ͬ����������ҵ縺�����ʵ縺�ԣ�O��N��C����ԭ�ӵĺ˵����Ϊ7����������Ų�ʽΪ1s22s22p3������Χ�����Ų�ʽ��2s22p3��
��2��H2O2Ϊ���ۻ���������д��������������һ��O-O��������ʽΪ��![]() ��O-H��O-O����Ϊ�Ҽ�����H2O2�Ľṹ��֪��Oԭ���γ�1��O-H����1��O-O��������2�Թ¶Ե��ӣ��ӻ������Ϊ4���ӻ���ʽΪsp3��ˮΪ���Է��ӣ�H2O2��ˮ���ܣ����Է��������ڼ��Է����У�H2O2�������ǻ��ϣ���ԭ������һ�����ӵ���ԭ�����γ������
��O-H��O-O����Ϊ�Ҽ�����H2O2�Ľṹ��֪��Oԭ���γ�1��O-H����1��O-O��������2�Թ¶Ե��ӣ��ӻ������Ϊ4���ӻ���ʽΪsp3��ˮΪ���Է��ӣ�H2O2��ˮ���ܣ����Է��������ڼ��Է����У�H2O2�������ǻ��ϣ���ԭ������һ�����ӵ���ԭ�����γ������
��3��OCN-����3��ԭ�ӣ��۵�������Ϊ6+4+5+1=16������ȵ�����ΪCO2����N2O���ȣ�����OCN-��Ϊ�ȵ���������У��ɵ�Ԫ����ɵ���������N3-��
��4��KCN�ľ����к�K����Ϊ12![]() +1=4��CN-��8��
+1=4��CN-��8��![]() +6��
+6��![]() =4��������K����Χ�����CN-��6����������K������λ��Ϊ6�����������Ϊa3=(0.648��10-7)3cm3��1NA����������Ϊ4����39+12+14��g=4��65g����KCN ������ܶ�Ϊ
=4��������K����Χ�����CN-��6����������K������λ��Ϊ6�����������Ϊa3=(0.648��10-7)3cm3��1NA����������Ϊ4����39+12+14��g=4��65g����KCN ������ܶ�Ϊ![]() g/cm3��
g/cm3��

