��Ŀ����
����Ŀ��
��1����֪����CO��g��+ ![]() O2��g��=CO2��g����H=-283.0kJmol-1
O2��g��=CO2��g����H=-283.0kJmol-1
��CH3OH��l��+ ![]() O2��g��=CO2��g��+2H2O��l����H=-726.5kJmol-1
O2��g��=CO2��g��+2H2O��l����H=-726.5kJmol-1
��д���״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽ�� ��
��2����ѧ���ѻ���˼��������о������N4���ӣ���ṹΪ�������壨��ͼ��ʾ��������������ƣ���֪����1molN-N������193kJ����������1molN��N������941kJ��������1molN4����ת��Ϊ2molN2ʱ�ġ�H= ��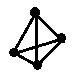
���𰸡�
��1��CH3OH��l��+O2��g���TCO��g��+2H2O��l����H=-443.5kJ?mol-1
��2��-724kJ?mol-1
����������1����֪����CO��g��+ ![]() O2��g��=CO2��g����H=-283.0kJmol-1 ����CH3OH��l��+
O2��g��=CO2��g����H=-283.0kJmol-1 ����CH3OH��l��+ ![]() O2��g��=CO2��g��+2H2O��l����H=-726.5kJmol-1 ���ڣ��ٵ�CH3OH��l��+O2��g���TCO��g��+2H2O��l�������H= -726.5kJmol-1 -��-283.0kJmol-1��= -443.5kJmol-1 ���״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽΪCH3OH��l��+O2��g���TCO��g��+2H2O��l����H=-443.5kJmol-1��
O2��g��=CO2��g��+2H2O��l����H=-726.5kJmol-1 ���ڣ��ٵ�CH3OH��l��+O2��g���TCO��g��+2H2O��l�������H= -726.5kJmol-1 -��-283.0kJmol-1��= -443.5kJmol-1 ���״�����ȫȼ������һ����̼��Һ̬ˮ���Ȼ�ѧ����ʽΪCH3OH��l��+O2��g���TCO��g��+2H2O��l����H=-443.5kJmol-1��
��2����1molN4����ת��Ϊ2molN2ʱ��Ҫ����6molN-N�����γ�2molN��N������֪����1molN-N������193kJ����������1molN��N������941kJ��������1molN4����ת��Ϊ2molN2ʱ�ġ�H=��193 ![]() -941
-941 ![]() ��kJmol-1 = -724kJmol-1��
��kJmol-1 = -724kJmol-1��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ����ˮ���Ȼ���(SnCl4)�������л��ϳɵ��Ȼ�������ʵ���ҿ������ڵ���(�۵�231.9C)��Cl2 ��Ӧ�Ʊ�SnCl4װ������ͼ��ʾ��
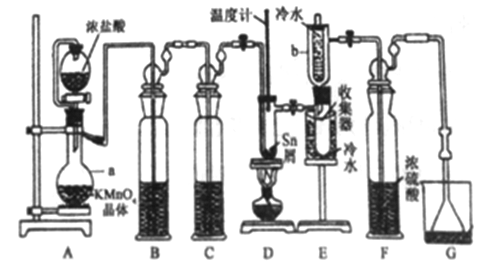
��֪���� SnCl4�ڿ����м���ˮ������SnO2xH2O����SnCl2��SnCl4�й������������±���
���� | ��ɫ��״̬ | �۵�/�� | �е�/�� |
SnCl2 | ��ɫ���� | 246 | 652 |
SnCl4 | ��ɫҺ�� | -33 | 114 |
�ش�����������
��1������a��������__________�����з�����Ӧ�����ӷ���ʽΪ________��
��2��װ��B��C ��ʢװ���Լ��ֱ���_______��__________��
��3�����۲쵽װ��FҺ���Ϸ�����_____����ʱ�ſ�ʼ��ȼ�ƾ��ƣ������ۻ����ʵ����������������������ȡ���ʱ�������ȵ�Ŀ���������ӿ�����������Ӧ����__________��
��4��Eװ����b��������___________��
��5�����Ƶò�Ʒ�к�������Cl2����ɲ�������_____(����ĸ)��ʩ���Գ�ȥ��
A.����NaOH ��ȡ��Һ B.�����������ټ�������
C.����⻯���������� D.���뱥��ʳ��ˮ��ȡ
��6�����õζ����ⶨ����Ʒ�Ĵ��ȡ�ȷ��ȡ����Ʒmg���뵽����Ũ�����ܽ⣬Ȼ���ټ�ˮϡ����250 mL������Һ����ȡ25.00 ml��ϡ�ͺ����Һ����ƿ�У��������ε�����Һ��ָʾ�ƣ���cmol/L ��KIO3 ����Һ���еζ���ƽ�еζ�3 �Σ�ƽ������V mL�ı���Һ����֪�ζ�ʱ�����ķ�ӦΪ:Sn2++IO3-+H+��Sn4++I2+H2O(δ��ƽ)���ش�����������
���жϴﵽ�ζ��յ������Ϊ____________��
����Ʒ��SnCl2(Ħ������ΪM g/mol )�ĺ���Ϊ____%(�ú�m��c��V��M �Ĵ���ʽ��ʾ)��