��Ŀ����
18��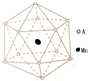 �������Ϊ�����⾧����һ�ֽ��ھ���ͷǾ���֮��Ĺ���ṹ��
�������Ϊ�����⾧����һ�ֽ��ھ���ͷǾ���֮��Ĺ���ṹ����1����һ��������ͭ��������Ԫ�ع��ɵ���Ȼ���廯���������������ɵ�һ�ּ������Ƚ���������з��飬Ȼ����������ʵ�飺
��1������ȡ38.99g ���壬������������NaOH��Һ��ַ�Ӧ���ڱ�״���£��ռ��õ�21.84L ������
��2������1����Ӧ�����Һ���˳����壬������ˮϴ�ӣ���ε���6mol/L���ᣬ���������������Ϊ40mLʱ�����岻���ܽ⣮
���������ĵ�һ�����ܵĴ�С��ϵΪFe��Al����Ԫ�ط��ű�ʾ����
�ڸ�������ʵ���ȷ��������Ļ�ѧʽΪAl65Cu23Fe12��
��2������һ�ֶ�����Ԫ��A�γɵ�����̬��һ��������֪A�����������������Ӳ����������к��гɶԺͲ��ɶԵ��ӣ�
����ԭ���ڻ�̬ʱ�ĺ�������Ų�ʽΪ[Ar]3d64s2��
������A�������еĽ����Ϊ�������������Ӽ������ۼ��������������Ӽ���������
������A�γɵ����ṹ��ͼ��ʾ���������Ļ�ѧʽΪMnAl12��
�ܹ��ɽ��������Mn2��CO��n������ԭ�Ӽ۵������������ṩ��������֮��Ϊ34����n=10��
���� ��1���ٽ�����Խǿ������Խ��ʧ���ӣ���һ������ԽС��
����������NaOH��Һ��ַ�Ӧ��ֻ��Al������������Һ��Ӧ�������������Al�����ʵ�����������ʣ���������ᣬ���е�Fe�����ᷴӦ��������������ʵ������Fe�����ʵ����������������������Cu���������������ʵ���֮�ȵ���ԭ�Ӹ����ȼ��㣻
��2����֪A�����������������Ӳ����������к��гɶԺͲ��ɶԵ��ӣ�������2�����Ӳ㣬��ΪBe��Beû��δ�ɶԵ��ӣ������ϣ��������������Ӳ㣬��ΪAlԪ�أ�Al���гɶԺͲ��ɶԵ��ӣ����ϣ�����AΪAlԪ�أ�
��MnΪ25��Ԫ�أ�������25�����ӣ����ݹ���ԭ����д��
��Mn��Al�γɵĺϽ��д��ڽ�������
�۸���������Mnԭ����Alԭ�Ӹ����жϣ�
������Mn�ļ۵�������CO�ṩ���Ӷ�������ó�������ԭ������ԭ�ӣ���۵�������7��ÿ�������ṩ�ĵ�������2��
��� �⣺��1���ٽ�����Խǿ������Խ��ʧ���ӣ���һ������ԽС��������Al��Fe�����һ�����ܣ�Fe��Al��
�ʴ�Ϊ��Fe��Al��
����������NaOH��Һ��ַ�Ӧ��ֻ��Al������������Һ��Ӧ����֪����21.84L ��������n��H2��=$\frac{21.84L}{22.4L/mol}$=0.975mol����n��Al��=$\frac{2}{3}$n��H2��=0.65mol��
ʣ���������ᣬ���е�Fe�����ᷴӦ����������ʵ���Ϊn��HCl��=6mol/L��0.04L=0.24mol����n��Fe��=0.12mol��
m��Cu��=38.99-0.65��27-0.12��56=14.72g����n��Cu��=$\frac{14.72g}{64g/mol}$=0.23mol��
��ѧʽ��ԭ�Ӹ����ȣ�N��Al����N��Cu����N��Fe��=0.65mol��0.23mol��0.12mol=65��23��12��������ʵĻ�ѧʽΪAl65Cu23Fe12��
�ʴ�Ϊ��Al65Cu23Fe12��
��2����֪A�����������������Ӳ����������к��гɶԺͲ��ɶԵ��ӣ�������2�����Ӳ㣬��ΪBe��Beû��δ�ɶԵ��ӣ������ϣ��������������Ӳ㣬��ΪAlԪ�أ�Al���гɶԺͲ��ɶԵ��ӣ����ϣ�����AΪAlԪ�أ�
��MnΪ25��Ԫ�أ�������25�����ӣ����ݹ���ԭ����д�����Ų�ʽΪ��[Ar]3d64s2��
�ʴ�Ϊ��[Ar]3d64s2��
��Mn��Al�γɵĺϽ��н���ԭ��֮��ͨ����������ϣ�
�ʴ�Ϊ����������
����ͼ��֪�����к���һ��Mnԭ�Ӻ�12��Alԭ�Ӹ������������Ļ�ѧʽΪMnAl12��
�ʴ�Ϊ��MnAl12��
������ԭ������ԭ�ӣ���۵�������7��ÿ�������ṩ�ĵ�������2����7��2+2n=34����n=10��
�ʴ�Ϊ��10��
���� ���⿼�������ʻ�ѧʽ��ȷ������һ�����ܡ������Ų�ʽ����λ���ȣ���Ŀ�漰��֪ʶ��϶࣬��Ŀ�Ѷ��еȣ������ڿ���ѧ���Ի���֪ʶ���ۺ�Ӧ��������

| A�� | ����CH3-CHOH-COOH | |
| B�� | �������ǡ�CH2OH-CHOH-CHOH-CH2-CHO | |
| C�� | ����CH2OH-CHOH-CH2OH | |
| D�� | ���ǡ�CH2OH-CHOH-CHOH-CHOH-CHO |
| A�� | ����5���Ҽ���1���м� | |
| B�� | C-H֮����̼��sp2�ӻ������H��s����γɵĦҼ���C-C֮����δ�μ��ӻ���2p����γɵĦм� | |
| C�� | ֻ��sp2�ӻ�������γɦм� | |
| D�� | C-C֮����sp2�γɵĦҼ���C-H֮����δ�μ��ӻ���2p����εĦҼ� |
| A�� | sp1 | B�� | sp2 | C�� | sp2 | D�� | sp3 |
| ѡ�� | ʵ��Ŀ�� | ��������Ʒ |
| A | �����Ҵ������������Ļ���� | ��Һ©�����ձ��������� |
| B | ������Һ���Ƿ���SO42- | �Թܡ���ͷ�ιܡ�Ba��NO3��2 |
| C | SO2���������ԣ����л�ԭ�� | �Թܡ���ͷ�ιܡ���ˮ��Ʒ�� |
| D | ����100mLpH=2������ | 100mL����ƿ���ձ�������������ͷ�ιܡ���ʽ�ζ��ܡ�pH=1������ |
| A�� | A | B�� | B | C�� | C | D�� | D |
| A�� | ƽ����Ӧ�ٶȣ��ң��� | B�� | ƽ��ʱSO3�����ʵ����ף��� | ||
| C�� | ƽ��ʱSO2��ת���ʣ��ң��� | D�� | ƽ��ʱ���������ܶȼף��� |
| A�� | ���죬��״��п��2mol/Lϡ������Һ��Ӧ | |
| B�� | ���죬��ĩ״��п��2mol/Lϡ������Һ��Ӧ | |
| C�� | ���죬��״��п��2mol/Lϡ������Һ��Ӧ | |
| D�� | ���죬��ĩ״��п��2mol/Lϡ������Һ��Ӧ |
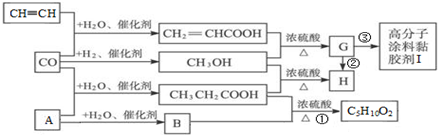
 ��
��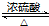 CH3CH2COOC2H5+H2O��ָ���ڵķ�Ӧ���ͼӳɷ�Ӧ��
CH3CH2COOC2H5+H2O��ָ���ڵķ�Ӧ���ͼӳɷ�Ӧ��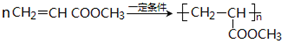 ��
�� ��Ȼ������Ҫ�ɷ��Ǽ��飬�����������ʻ���COS��������C2H5SH�������壮
��Ȼ������Ҫ�ɷ��Ǽ��飬�����������ʻ���COS��������C2H5SH�������壮 ��
��