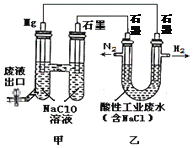
��Ŀ����
����Ŀ���״�(CH3OH)����Ҫ���ܼ������ȼ�ϣ���ҵ�ϳ���CO��H2�Ļ������Ϊԭ����һ���������Ʊ��״���
(1)��ҵ�Ͽ���ͨ������;�����H2�����н���Ч����õ���___________��
A.���·ֽ�ˮ��ȡH2:2H2O![]() 2H2��+O2��
2H2��+O2��
B.���ˮ��ȡH2:2 H2O![]() 2H2��+ O2��
2H2��+ O2��
C.������ˮ��Ӧ��ȡH2:CH4+ H2O![]() 3H2+CO
3H2+CO
D.�ڹ���������£�����̫���ֽܷ�ˮ��ȡH2:2H2O![]() 2H2��+ O2��
2H2��+ O2��
(2)��2L���ܱ������г���1mo1 CO��2mol H2��һ�������·�����Ӧ;CO(g)+2H2(g) ![]() CH3OH(g)�����CO��CH3OH(g)�����ʵ�����ʱ��ı仯����ͼ��ʾ��
CH3OH(g)�����CO��CH3OH(g)�����ʵ�����ʱ��ı仯����ͼ��ʾ��
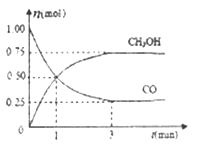
�ٴӷ�Ӧ��ʼ��ƽ�⣬������ƽ����Ӧ����v(H2)=_____��ƽ��ʱ�������������Ϊ____��
���ܹ��жϸ÷�Ӧ�ﵽƽ�����______��
A. CO��H2��CH3OH�������ʵ�Ũ�����
B. �ܱ������л��������ܶȲ��ٸı�
C. �ܱ������л�������ƽ����Է����������ٸı�
D. ��ͬʱ��������1mo1CO��ͬʱ����1mol CH3OH
(3)Ϊʹ�ϳɼ״�ԭ�ϵ�ԭ�������ʴﵽ100%��ʵ���������Ʊ�ˮú��ʱ����ʹ��CH4��������Ͷ��ʱ��n(C):n(H2O):n(CH4)=__________��
(4)�ݱ��������Ħ��������˾�з���һ���ɼ״��������Լ�ǿ�����������Һ�������ֻ���أ����������������غ�﮵�ص�10����������ʹ��һ���²ų��һ�Ρ��ٶ��ŵ�����У��״���ȫ���������Ķ�����̼�������������CO32-����õ�ظ����ĵ缫��ӦʽΪ__________________����طŵ��������Һ��pH��_____(�����½�����������������������)��
���𰸡�D 0.25 molL-1min-1 33.3% CD 1��2��1 CH3OH - 6e- + 8OH- =CO32-+6H2O �½�
��������
��1��ͨ����ͬ;�����H2�����Լ��Դ��������̫���ܣ�
��2���ٸ���![]() ����v��CO�����ٸ�������֮�ȵ���ϵ��֮�ȼ���v��H2������������ʽ�������������������
����v��CO�����ٸ�������֮�ȵ���ϵ��֮�ȼ���v��H2������������ʽ�������������������
��ƽ���־�����淴Ӧ������ͬ������ֺ������䣻
��3��Ϊʹ�ϳɼ״�ԭ�ϵ�ԭ�������ʴ�100%��������n��C����n��H����n��O��=1��4��1���ݴ˷�����
��4���ŵ�ʱ������ӦΪ��CH3OH-6e-+8OH-=CO32-+6H2O�������缫��Ӧʽ��O2+2H2O+4e-=4OH-����ط�ӦΪ2CH3OH+3O2+4OH-=2CO32-+6H2O���ݴ˷�������
��1��ͨ����ͬ;�����H2�����Լ��Դ��������̫���ܣ���������ѡ���������ܡ����ܣ�������Ҫ�ʴ�Ϊ��D��
��2���ٴӷ�Ӧ��ʼ��ƽ�⣬��n(CO)=1mol0.25mol=0.75mol����v(CO)=![]() =0.125mol/(Lmin)���ɷ���ʽ�Ŀ�֪v(H2)=2v(CO)=2��0.125mol/(Lmin)=0.25mol/(Lmin)��
=0.125mol/(Lmin)���ɷ���ʽ�Ŀ�֪v(H2)=2v(CO)=2��0.125mol/(Lmin)=0.25mol/(Lmin)��
�г�����ʽ���£�
 �����������������=
�����������������=![]() =
=![]() =33.3%���ʴ�Ϊ��0.25mol/(Lmin)��33.3%��
=33.3%���ʴ�Ϊ��0.25mol/(Lmin)��33.3%��
��A. CO��H2��CH3OH�������ʵ�Ũ����Ȳ�һ���ﵽƽ�⣬A�����
B. ��Ӧǰ���������䣬���Ҳ���䣬���ܱ������л��������ܶ�һֱ���䣬���Բ�һ���ﵽƽ�⣬B�����
C. �ܱ��������������䣬��������ƽ����Է����������ٸı䣬˵���ܵ����ʵ������䣬��÷�Ӧ�������������С����ϵ���ʻ�������ƽ����Է����������ٸı䣬��˵����Ӧ�ﵽƽ�⣬C����ȷ��
D. ��ͬʱ��������l mol CO��ͬʱ����1molCH3OH�������淴Ӧ������ȣ���˵����Ӧ�ﵽƽ�⣬D����ȷ��
�ʴ�Ϊ��CD��
��3��Ϊʹ�ϳɼ״�ԭ�ϵ�ԭ�������ʴ�100%,������n(C):n(H):n(O)=1:4:1,����n(C):n(H2O):n(CH4)=1:2:1���ʴ�Ϊ��1:2:1��
��4����1���ŵ�����У��״���ȫ����������CO2�������������CO32-����õ�ظ����ĵ缫��ӦʽΪCH3OH - 6e- + 8OH- =CO32-+6H2O��
��2���ŵ�ʱ���״���������Ϊ��ظ�����Ӧ�����������������õ��ӵĻ�ԭ��Ӧ���䷴Ӧ���ܷ���ʽΪ��2CH3OH+3O2+4OH-=2CO32-+6H2O����֪��������������OH-���������ҺpH���½���